| |
Intravenous immunoglobulin 1 g/kg as the
initial treatment for Kawasaki disease
Hirohiko Shiraishi, Mayu Iino, Masaru Hoshina, Kou Ichihashi, Mariko Y Momoi
Tochigi, Japan
Author Affiliations: Department of Pediatrics, Jichi Medical University, Tochigi, 329-0498, Japan (Shiraishi H, Iino M, Hoshina M, Ichihashi K, Momoi MY)
Corresponding Author: Hirohiko Shiraishi, MD, Department of Pediatrics, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke, Tochigi, 329-0498, Japan (Tel: +81-285-58-7366; Fax: +81-285-44-6123; Email: shiraish@jichi.ac.jp)
Background: Coronary artery lesion (CAL) in Kawasaki disease (KD) is prevented by intravenous immunoglobulin (IVIG); however, the total amount of IVIG should be reduced if the outcome is the same. Our aim was to determine whether the treatment with IVIG at an initial dose of 1 g/kg on the 5th to 7th day of illness with additional IVIG for refractory patients is effective for preventing CAL.
Methods: A total of 107 KD patients were treated according to the days of illness and the Harada score within 7 days of illness. All the patients with Harada score 4 or more were treated with IVIG at an initial dose of 1 g/kg, and the patients who were refractory to the initial dose, additional IVIG at a dose of 1 g/kg up to 3 g/kg was infused. Echocardiography was performed to detect the incidence of CAL.
Results: Seventy-eight patients (73%) were treated with IVIG at an initial dose of 1 g/kg according to the Harada score and the duration of illness; IVIG was started when their Harada score became 4 or more and basically on the 5th day or later. Six critically ill patients were treated with IVIG at a dose of 1 g/kg starting from the 2nd or 4th day, and all of them were refractory to the initial dose of 1 g/kg and further treated with additional doses of 1 to 3 g/kg (CAL was not observed); whereas the other 72 patients (of whom 42 were admitted by the 4th day and waited until the 5th day) were treated on the 5th to 7th day with IVIG at an initial dose of 1 g/kg. Of the 78 patients, 57 responded to the initial dose of 1 g/kg, but the remaining 21 refractory patients required additional IVIG (a total dose of IVIG up to 4 g/kg). Twenty-nine patients (27%) were treated without IVIG because their Harada score was less than 4, and CAL was not observed. In 4 (3.7%) of the 107 patients who had IVIG at 1 g/kg (n=1) or additional IVIG up to 3 g/kg (n=3), CAL appeared but regressed within 6 months after the onset.
Conclusion: Treatment of KD with IVIG at an initial dose of 1 g/kg on the 5th to 7th day with additional IVIG for refractory patients can have the same effect as the standard protocol (IVIG of 2 g/kg).
Key words: coronary artery; echocardiography; immunoglobulin; Kawasaki disease
World J Pediatr 2007;3(3):195-199
Introduction
Kawasaki disease (KD) is associated with coronary artery aneurysm in 15% to 25% of patients.[1] Acute phase KD is treated with aspirin and intravenous immunoglobulin (IVIG), and the incidence of coronary artery aneurysm has decreased after the introduction of high-dose IVIG.[2,3] The standard care for children with acute phase KD is treatment with a single infusion of high-dose (2 g/kg) IVIG within the 10th day of illness and aspirin.[4] Although a dose-dependent effect is reported in the treatment of KD,[5] the total amount of IVIG should be reduced if the incidence of coronary artery lesions (CALs) can be the same as that with the standard protocol. Also, it is reported that early treatment of KD with IVIG (on the 1st to 4th day of illness) is likely to require additional IVIG.[6,7] In the treatment of KD, we found that IVIG at a dose of 1 g/kg was effective in most patients when it was infused on the 5th to 7th day of illness. Thus, we began to treat KD with this regimen from February 2002. The aim of this study was to assess the efficacy of IVIG at an initial dose of 1g/kg on the 5th to 7th day of KD with additional IVIG in refractory patients, and whether the total amount of IVIG is reduced when patients were selected by the Harada score.
Methods
In this study, we retrospectively reviewed clinical records of consecutive 118 acute phase KD patients who were admitted to our institute between February 2000 and April 2004. Our criteria for a diagnosis of KD included fever (temperature exceeding 38 degrees Celcius) accompanied by the presence of at least 4 of the following 5 findings: bilateral conjunctival injection, changes in the lips and oral cavity, nonpurulent cervical lymphadenopathy, polymorphous exanthema, and changes in the extremities. These diagnostic criteria were consistent with the Diagnostic Guidelines for Kawasaki Disease (5th revision).[8] Incomplete KD was defined as lacking sufficient clinical signs of the disease to fulfill the above criteria.[4] The first day of the illness was defined as the first day of fever. Fourteen patients who were admitted or treated beyond the 7th day of illness were excluded in this study. The total number of KD patients who were admitted within the 7th day of illness was 104, among whom 3 patients had recurrent KD (there were 14, 18 and 34 months between the initial and the second episode of KD, respectively) during this period. Thus, a total of 107 admissions were enrolled in this study. Ninety-six patients met the criteria of KD, and 11 patients were diagnosed as having incomplete KD. There were 74 male (two of them had recurrent KD) and 30 female patients (one of them had recurrent KD). In this report, one admission for KD is counted as one patient because the clinical data and CAL of each patient were not substantially different between the initial and the second admissions. The average age at the onset of KD was 2.47 (range 0.16 to 8.85) years. In this study there was no control group, and therefore no statistical analysis was performed.
Laboratory data
Complete blood count, C-reactive protein (CRP), serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and albumin were examined at least on admission, at 36 to 48 hours after completion of IVIG, and before discharge.
Treatment
Once the patients were diagnosed as having KD, they were treated with oral aspirin at 30 mg/kg per day, or dipyridamole at 5 mg/kg per day when the AST or ALT level was above 100 mU/ml. Then, the medication was changed to oral aspirin at 5 mg/kg per day when the body temperature was reduced to below 37.5 degrees Celcius and AST and ALT levels were reduced to °‹100 mU/ml. The guidelines established by Harada (Harada score) were used to select those KD patients who were likely to develop CALs; IVIG was started when the patient had Harada score of 4 or more (Table).[9]
Table. Harada score
When the patient with KD satisfies 4 or more of the above 7 criteria within the 9th day of illness, treatment with IVIG should be started.
|
1) WBC count: equal to or more than 12 °Ń103/¶Ől |
|
2) Platelet count: less than 35°Ń104/¶Ől |
|
3) CRP: equal to or more than 4 mg/dl |
|
4) Hematocrit: less than 35% |
|
5) Serum albumin: less than 3.5 g/dl |
|
6) Gender: male |
|
7) Age: equal to or less than 12 months |
Fig. IVIG treatment for KD according to the day of illness and Harada score. Patients were treated according to the duration of KD and Harada score. Add IVIG: additional intravenous immunoglobulin; CAL: coronary artery lesion; Harada: Harada score; KD: Kawasaki disease; IVIG: intravenous immunoglobulin; Refractory: patients who were refractory to the initial treatment; Responder: patients who responded to the initial treatment.
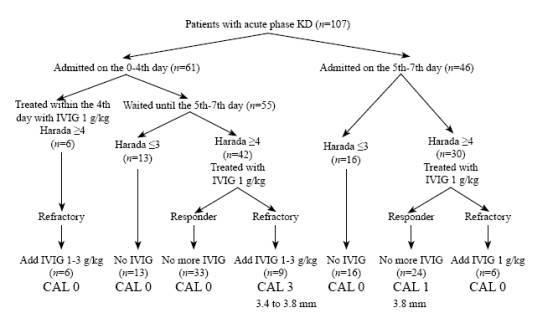
Our basic treatment strategy for KD was as follows (Fig.).
1) When admitted before the 5th day of illness, the patient was asked to wait until the 5th day of illness without IVIG, even if the patient's Harada score was °›4. If the patient was critically ill and suspected of having meningitis or hyponatremia was present, IVIG at a dose of 1 g/kg was started on admission. On the 5th to 7th day of illness, if the patient's Harada score was °‹3, no IVIG was infused, and if their Harada score was °›4, IVIG at 1 g/kg was infused for over 10 hours in one day.
2) When the patient was admitted on the 5th to 7th day of illness and the patient's Harada score was °‹3, no IVIG was infused. They were asked to wait until the end of the 7th day of illness, and once the Harada score became °›4, IVIG at 1 g/kg was started. When the patient's Harada score on admission was °›4, IVIG at 1 g/kg was infused.
3) For those patients who were refractory to the initial IVIG, additional IVIG at 1 g/kg was infused; for those who were refractory to the second IVIG, total IVIG up to 4 g/kg was infused. The patients who were refractory to IVIG were defined as having fever (°›37.5 degrees Celcius), increased white blood cell counts (WBC), or poor CRP decrease (more than half of the pre-treatment level) that was observed 36 to 48 hours after the end of the initial IVIG. Those patients who fulfilled all the 3 parameters were considered to be responders, and IVIG treatment was considered to be effective.
Several IVIG preparations were used for the treatment of KD in this study: polyethylene glycol-treated human immunoglobulin (Venoglobulin IH®, Mitsubishi-Welpharma Ltd) in 46 patients, pH4 stabilized acid human immunoglobulin (Poliglobin N®, Bayer Yakuhin Ltd) in 25 patients, and sulfonated human immunoglobulin (Venilon®, Teijin Ltd) in 7 patients. Basically, the same preparation was used for the additional IVIG, except for 1 patient who showed an allergic response to the second IVIG, and the third IVIG preparation was changed to another preparation (from Poliglobin N® to Venoglobulin IH®).
Echocardiography
The initial echocardiography was performed before IVIG treatment, and subsequent echocardiography was performed after IVIG, before discharge, and 1 month after the onset of KD. CAL was defined and classified as follows: coronary artery ectasia, when the coronary artery was dilated with a diameter °‹4 mm or when the diameter was less than 1.5 times that of the adjacent artery diameter; and coronary artery aneurysm, when the coronary artery was dilated with a diameter >4 mm. Once CAL was observed, oral aspirin was continued and follow-up echocardiography was performed until it disappeared. Coronary artery dilatation or aneurysm observed within 1 month was defined as CAL in this study.
Results
A total of 107 KD patients were admitted and treated within the 7th day of illness during the study period (Fig.).
Sixty-one patients were admitted before the 5th day of illness. Among them, 6 patients were treated before the 5th day of illness (2 to 4 days) and showed Harada scores °›4 before IVIG therapy. They were refractory to IVIG (5 of them had persistent fever, and all had poor CRP decrease) and were treated with additional IVIG (1 g/kg in 2 patients, 2 g/kg in 3 patients and up to 3 g/kg in 1 patient), but no CAL was observed. Fifty-five patients were asked to wait until the 5th to 7th day of illness. Among them, 13 patients were treated without IVIG because their Harada scores were °‹3, and no CAL was observed. In the other 42 patients treated with IVIG at 1 g/kg on the 5th to 7th day of illness, 33 responded to the initial IVIG. One patient who had febrile convulsion on the second day of illness and was treated with dexamethasone, and who responded to the initial IVIG, was included. Nine patients, however, were refractory to the initial dose of IVIG at 1 g/kg and were treated with additional IVIG (1 g/kg in 6 patients, 1.6 g/kg in 1, 2 g/kg in 1, and up to 3 g/kg in 1). CALs were observed in 3 of the 9 patients, who received total IVIG of 2 g/kg, 2.6 g/kg and 4 g/kg for coronary artery ectasia of 3.4 mm, 3.8 mm and 3.8 mm, respectively (Fig.).
Forty-six patients were admitted on the 5th to 7th day of illness. Sixteen patients were treated with no IVIG because their Harada scores were °‹3, and became defervescent spontaneously, without CAL appearance. In the other 30 patients with Harada scores °›4 and treated with IVIG at 1 g/kg on the 5th to 7th day of illness, 24 responded to the initial IVIG, including 1 patient developed CAL (coronary artery ectasia of 3.8 mm); 6 patients were refractory to the initial IVIG and treated with additional IVIG at 1 g/kg, but no CAL was observed (Fig.).
Eleven patients (10%) who were diagnosed as having incomplete KD because of lacking sufficient clinical signs of the disease to fulfill the criteria were included in this study. The average age at the onset of incomplete KD was 2.14 (range 0.16 to 5.28) years. Eight of the 11 patients were treated without IVIG and 3 were treated with IVIG at 1 g/kg on the 6th day of illness, but no CAL was observed.
In the 78 patients (73%) who were treated with IVIG at an initial dose of 1 g/kg, 6 were treated before the 4th day of illness, and 72 on the 5th to 7th day. Of these 78 patients, 57 (73%) responded to a single infusion of IVIG at a dose of 1 g/kg, and 21 (27%) required re-treatment or multiple infusions of IVIG (total IVIG up to 4 g/kg). CALs were observed in 4 patients who had been infused with total IVIG at a dose of 1 g/kg, 2 g/kg, 2.6 g/kg and 4 g/kg, respectively. All CALs regressed spontaneously within 6 months after the onset of KD, as observed echocardiographically. In 29 patients (27%) whose Harada scores were °‹3 and were treated without IVIG, no CAL was observed.
In this study, the total amount of IVIG used in the 78 patients was 1275.5 g: IVIG 1 g/kg in 57 patients, 2 g/kg in 14, 2.6 g/kg in 1, 3 g/kg in 3, and 4 g/kg in 3. Total body weight of the 107 patients was 1325.5 kg. Side-effect of IVIG was observed in 1 patient, whose second infusion of IVIG was stopped midway (0.6 g/kg), and the patient was treated with another preparation on the next day (a total dose of IVIG was 2.6 g/kg). Total IVIG up to 4 g/kg was infused in 3 patients, but no dose-related side effects were observed.
Discussion
In this study, the IVIG therapy at an initial dose of 1 g/kg on the 5th to 7th day of illness and additional IVIG for the refractory patients was effective. The incidence of CAL was lower than that reported in a nationwide KD survey in which most of the patients were treated with the standard protocol (IVIG of 2 g/kg).[4] The IVIG therapy at an initial dose of 1 g/kg could reduce the total amount of IVIG used in treating acute phase KD.
The use of a moderate dose of IVIG for KD has been reported by Khowsathit et al.[10] They started to treat KD patients with IVIG at a dose of 1 g/kg, and the response rate (76%) was similar to that in our study. They treated KD patients with IVIG up to 3 g/kg; however, the efficacy of preventing CAL was lower than that of the high-dose regimen (2 g/kg). In our series, the patients who were refractory to the initial IVIG dose of 1 g/kg, IVIG of 2 g/kg could be infused within the 9th day, and the dose up to 4 g/kg was infused. The incidence of CAL at 1 month (4 in 107 patients; 3.7%) was the same as that in a nationwide KD survey, in which most of the patients were treated with IVIG at a dose of 2 g/kg.[11]
Six patients who had KD for less than 4 days were treated with IVIG at 1 g/kg, but failed. They were successfully retreated with additional IVIG (a total dose up to 4 g/kg). Early treatment of KD with IVIG at a dose of 2 g/kg is reported to be associated with persistent/recrudescent fever that required additional IVIG in 33% of the patients.[6] In our 6 patients, 5 had persistent fever and all had a poor CRP decrease, and were treated with additional IVIG. The high rate (100%) of additional IVIG in our 6 patients may be due to the shortage of the initial dose of IVIG. Muta et al[7] reported that early treatment is likely to result in a greater requirement for additional IVIG, which is supported by our results. In those critically ill patients who need to be treated on °‹ the 4th day of illness, an initial IVIG dose of 2 g/kg would be better than 1 g/kg.
We treated the patients who were refractory to the initial dose of 1 g/kg with additional IVIG. The patients with KD who were refractory to IVIG were treated with high-dose methylprednisolone,[12,13] low-dose methotrexate,[14,15] or ulinastatin.[16] Hashino et al[12] reported that steroid pulse therapy was given to those who were resistant to IVIG, but its efficacy for preventing CAL was not superior to that of additional IVIG therapy. Low-dose methotrexate therapy or ulinastatin might be promising, but only a few case reports have been published.[14,16] A randomized control study will disclose the efficacy of these therapies. In KD patients who are refractory to IVIG, plasma exchange therapy has been reported to be effective;[17] however, plasma exchange requires a rather complicated system, and cannot be easily utilized in general pediatric wards. Infliximab treatment for those refractory to IVIG was reported by Burns.[18] The total number of patients who were treated with this method is so small that the safety of this method has not been studied completely. In our series, the patients who were refractory to the initial IVIG were treated with additional IVIG (total IVIG up to 4 g/kg), but no patients suffered from dose-related side effects of IVIG, and no giant coronary aneurysms were observed.
Treating KD with IVIG was based on the score established by Harada (Table).[9] In our series, 78 patients were treated with IVIG, giving a rate of 73% close to that in Harada's report (73.4%).[9] The total amount of IVIG infused in our series was 1275 g: 1 g/kg in 57 patients, 2 g/kg in 14, 2.6 g/kg in 1, 3 g/kg in 3, and 4 g/kg in 3. If IVIG at a dose of 2 g/kg was given to all the patients (total body weight of 107 patients was 1325.5 kg), the total amount of IVIG would be 2651 g without taking account of the additional IVIG. Accordingly, at least 1376 g of IVIG was saved in our protocol. Sato et al[19] reported the cost effectiveness of high-dose IVIG in selected patients using the Harada score. In their study, IVIG at a dose of 2 g/kg was superior to the 5-day 400 mg/kg therapy. In this study, the same scoring system (Harada score) was used, but IVIG at a dose of 1 g/kg resulted in much less utilization of IVIG.
In 11 patients (10%) who were diagnosed as having incomplete KD, 8 patients were treated without IVIG and 3 with IVIG at a dose of 1 g/kg on the 6th day of illness; no CAL was observed. Incomplete KD is more common in young infants than in older children and the rate of incomplete KD is about 10%.[4,8] Even in patients with incomplete KD, CAL can develop; however, in this study the average age at the onset of incomplete KD was 2.14 (range 0.16 to 5.28) years, and CAL was prevented by a single infusion of IVIG at a dose of 1 g/kg on the 6th day in 3 patients. This might be explained by the benign nature of the condition in the incomplete KD patients enrolled in our study.
In our series, 4 patients (3.7%) had coronary artery ectasia at 1 month, which regressed within 6 months after onset. The total percentage of patients with CALs in the same period was 4.4 % in Japan, of whom most patients (79.8%) received IVIG at a dose of 2 g/kg.[8] Thus, our protocol showed the same effect on KD patients. This is a study that was performed in a single institute without controls, and a multicenter prospective randomized study would clarify the efficacy of our treatment strategy. The regimen for KD with IVIG at an initial dose of 1 g/kg on the 5th to 7th day with additional IVIG for refractory patients can be as effective as the standard protocol (2 g/kg). Accordingly, it can reduce the total amount of IVIG for KD patients without increasing CALs.
In conclusion, IVIG therapy for KD patients at an initial dose of 1 g/kg on the 5th to 7th day of illness and additional IVIG for the refractory patients was effective and the total amount of IVIG could be reduced when patients were selected by the Harada scoring system. This method can be an alternative for the prevention of CAL.
Funding: None.
Ethical approval: Not needed.
Competing interest: None declared.
Contributors: Shiraishi H proposed the study and wrote the first draft. Ichihashi K analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. Momoi MY is the guarantor.
References
1 Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94: 1379-1385.
2 Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984;2:1055-1058.
3 Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 1986;315: 341-347.
4 Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110:2747-2771.
5 Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr 1997;131:888-893.
6 Fong NC, Hui YW, Li CK, Chiu MC. Evaluation of the efficacy of treatment of Kawasaki disease before day 5 of illness. Pediatr Cardiol 2004;25:31-34.
7 Muta H, Ishii M, Egami K, Furui J, Sugahara Y, Akagi T, et al. Early intravenous gamma-globulin treatment for Kawasaki disease: the nationwide surveys in Japan. J Pediatr 2004; 144:496-499.
8 Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int 2005; 47:232-234.
9 Harada K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn 1991;33:805-810.
10 Khowsathit P, Hong-Hgam C, Khositseth A, Wanitkun S. Treatment of Kawasaki disease with a moderate dose (1 g/kg) of intravenous immunoglobulin. J Med Assoc Thai 2002;85 Suppl 4:S1121-1126.
11 Nakamura Y, Yashiro M, Uehara R, Yanagawa H. Results of the 17th nationwide survey on Kawasaki disease. Japan Kawasaki Disease Research Committee. J Pediatr Prac 2004; 67:313-323 (in Japanese).
12 Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int 2001;43:211-217.
13 Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr 1996;128:146-149.
14 Ahn SY, Kim DS. Treatment of intravenous immunoglobulin-resistant Kawasaki disease with methotrexate. Scand J Rheumatol 2005;34:136-139.
15 Lee MS, An SY, Jang GC, Kim DS. A case of intravenous immunoglobulin-resistant Kawasaki disease treated with methotrexate. Yonsei Med J 2002;43:527-532.
16 Iino M, Shiraishi H, Igarashi H, Honma Y, Momoi MY. Case of Kawasaki disease in NICU. Pediatr Int 2003;45:580-583.
17 Imagawa T, Mori M, Miyamae T, Ito S, Nakamura T, Yasui K, et al. Plasma exchange for refractory Kawasaki disease. Eur J Pediatr 2004;163:263-264.
18 Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr 2005;146:662-667.
19 Sato N, Sugimura T, Akagi T, Yamakawa R, Hashino K, Eto G, et al. Selective high dose gamma-globulin treatment in Kawasaki disease: assessment of clinical aspects and cost effectiveness. Pediatr Int 1999;41:1-7.
Received February 26, 2007 Accepted after revision June 12, 2007
|

