Separation and identification of endothelial progenitor cells from rat peripheral blood
Si-Lin Pan, Quan-Sheng Xing, Long Sun
Qingdao, China
Author Affiliations: Qingdao Children's Heart Center, Qingdao University Research Center for Congenital Heart Diseases, Qingdao 266011, China (Pan SL, Xing QS); Department of Cardiac Surgery, Qingdao Municipal Hospital, Qingdao 266011, China (Sun L)
Corresponding Author: Quan-Sheng Xing, Qingdao Children's Heart Center, Qingdao 266011, China (Tel: 86-13606426893; Fax: 86-532-82857650; Email: qsxing@163.com)
Background: Kawasaki disease (KD) as the most commonly acquired heart disease in children worldwide is an acute systemic febrile illness with endothelial injury. The incidence of KD varies in different countries, but it is higher in Asian than in Western countries. Endothelial progenitor cells (EPCs) can differentiate into endothelial cells and serve as a potential therapeutic agent for KD. The present study aimed at exploring the simple procedures of isolation, cultivation and purification of EPCs from peripheral blood.
Methods: Five milliliters of peripheral blood was collected from the femoral artery of each Sprague-Dawley rat. Mononuclear cells were isolated by Ficoll density gradient centrifugation and cultured in a special medium, including vascular endothelial growth factor and basic fibroblast growth factor. After 6 days of continuous culture on 24-well fibronectin-coated plates, the cells expanded and differentiated into endothelial-like progenitor cells. The expression of Flk-1, von Willebrand factor (vWF), CD31, and CD34 was assessed. Surface lectin staining was performed using fluorescently labeled UEA-1 lectin at 10 µg/ml. Meanwhile, the ability for live cells to take up fluorescently labeled acetylated low-density lipoprotein (DiI-Ac-LDL) was assessed by incubation with DiI-Ac-LDL.
Results: The cells harvested by this procedure were CD31, CD34, Flk-1 positive and demonstrated double-positive fluorescence for LDL, and lectin-UEA-1.
Conclusion: Relatively purified EPCs can be obtained by certain procedures of isolation and culture. For its differentiating potential, EPCs act as an important source of mature endothelial cells (ECs).
Key words: peripheral blood; mononuclear cell; endothelial progenitor cells; cell culture
World J Pediatr 2007;3(1):50-54
Introduction
Endothelial progenitor cells (EPCs) are considered to be derived from a common hematopoietic precursor cell with a high proliferative potential, and have thus been generally thought to contribute to vasculogenesis and repair of endothelial cells (EC). It has been reported that circulating EPCs can differentiate into endothelial cells and then exert effects on angiogenesis.[1] On the other hand, the latest study has demonstrated that Kawasaki disease (KD) patients associated with coronary artery lesion (CAL) have a large number of EPCs in the blood than those without CAL, and that circulating EPCs increase with delayed kinetics following the increase of circulating EC,[2] indicating that such EPCs are mobilized from the bone marrow into the circulation, and might be involved in the repair of EC injury and potential microneovascularization in KD vasculitis. Consequently, we hypothesize that EPCs play a significant role in KD recovery. The present study was undertaken to investigate the feasibility of obtaining EPCs from peripheral blood by certain procedures of isolation and cell culture, attempting to explore the clinical application in KD patients.
Methods
Mobilization of granulocyte colony-stimulating factor (G-CSF)
This study was approved by the Animal Research Committee of School of Medicine, Qingdao University. Male Sprague-Dawley rats were housed with their mothers under standard laboratory conditions until they were weaned for 21 days and their body weight was over 300 g. Recombinant human granulocyte colony-stimulating factor (rhG-CSF, Xiamen Tebao Bioengineering CO., LTD., 50 ¦Ìg/kg) or equivalent volume of saline was subcutaneously injected to determine its mobilizing effects on EPCs daily for 3 days. rhG-CSF was diluted with saline before injection so as to reduce the reading error.
Peripheral blood preparation
Three days after rhG-CSF administration, the rats were anesthetized with 1% of sodium phenobarbital (30 mg/kg, intraperitoneally) and supinely fastened onto the operation table. The femoral artery was exposed, and 5 ml blood was obtained through a trocar (22 G) with a 10 ml syringe, anticoagulated with 0.5 to 1.0 ml of heparin.
In vitro isolation, culture and expansion of EPCs
Blood from the femoral artery was diluted with 5 ml of Iscove's modified Dulbecco's medium (IMDM) (Gibco), and added to a tube with 5 ml of lymphocyte separation solution (¦Ñ=1.088, Huajing Biotech., Shanghai, China). The mixture was then centrifuged at 1500 rpm for 20 minutes, to separate into three layers. A buffy coat was left, mainly consisting of mononuclear cells (MNCs). The number of MNCs was counted under a phase-contrast microscope. Five randomly selected microscopic fields were evaluated, and the mean numbers of attaching cells and cell clusters were calculated in each blood sample. Thereafter, MNCs were transferred to another tube, diluted with IMDM and centrifuged for a second time at 1000 rpm for 5 minutes. The cells were washed, centrifuged and resuspended with IMDM. At a density of 1¡Á106/ml, the cells were cultured in IMDM, supplemented with human vascular endothelial growth factor (VEGF) (Sigma), basic fibroblast growth factor (bFGF) (Sigma), and fetal bovine serum (Gibco) on fibronectin-coated 24-well plates (BD, Biosciences Franklin Lakes, NJ) in the incubator at 37ºC and 5% CO2. Fresh media were added to the cultures every 3 days, and nonadherent cells were removed by washing with phosphate-buffered saline (PBS) solution. At days 3 and 5 of culture, the media were replaced.
Immunocytochemical analysis
For immunocytochemistry, differentiated MNCs were cultured overnight at 4ºC with the following primary antibodies: rabbit anti-rat Flk-1, rabbit anti-rat on von Willebrand factor (vWF), rabbit anti-rat CD31, and rabbit anti-rat CD34. Rabbit anti-rat F(ab')2 conjugated with fluorescein isothiocyanate (FITC) was used as the secondary antibody. Surface lectin staining was performed using fluorescently labeled UEA-1 lectin at 10 µg/ml. Meanwhile, the ability for live cells to take up fluorescently labeled acetylated low-density lipoprotein (DiI-Ac-LDL, Molecular Probes) was assessed by incubation with DiI-Ac-LDL (10 µg/ml) for 4 hours at 37ºC. After 4 days in culture, peripheral blood derived EPCs were extensively washed. Adherent cells on the 6th day of culture were stained by acetylated LDL, labeled with Di (DiI-Ac-LDL, Biomedical Technologies) and FITC-labeled lectin from ulex europaeus (Sigma), according to the manufacturer's instructions. After the staining, samples were analyzed under a confocal laser scanning microscope. The cells demonstrating double-positive fluorescence were identified as differentiating EPCs, as reported previously.[3,4] In fact, from the very beginning, expressions of vWF, Flk-1, CD34 and CD31 were analyzed daily for 6 days.
Statistical analysis
All data were presented as mean ¡À SD. Comparisons were performed by Student's t test between two values. A P value less than 0.05 was considered statistically significant.
Results
Mobilizing potentials of G-CSF on EPCs
Three days after rhG-CSF administration, MNC collection significantly increased in the mobilization group (2.5¡Á106/ml), as compared to the saline group (1.6¡Á106/ml) (P<0.01).
Immunofluorescence staining analysis
After 6 days culture of mononuclear cells, cobblestone- like adherent cells were observed (Fig. 1). Almost completely, the adherent cells were double stained by Di-I-Ac-LDL and FITC-labeled lectin (Figs. 2-4). These cells expressed endothelial cell-specific antigens (CD34 and CD31), which confirmed that the major population of adherent cells were EPCs. In fact, different expression levels of surface markers were observed. CD31 positive cells were increased from 27.0% ¡À 3.6% on day 2 to 94.0% ¡À 4.5% on day 4 (P< 0.05), and then decreased. Similarly, CD34 increased from 16.0% ¡À 4.2% on day 2 to 96.0% ¡À 3.5% on day 4, and then diminished. CD31 and CD34 disappeared on day 6. Both Flk-1 cells and vWF expression began to increase from day 3. Up to day 6, Flk-1 and vWF were expressed nearly on 100% of cells.
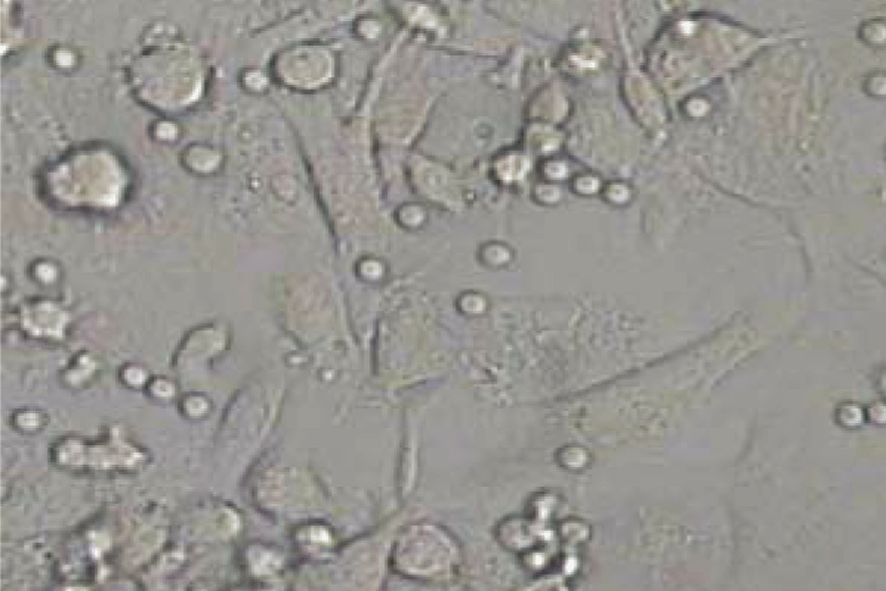
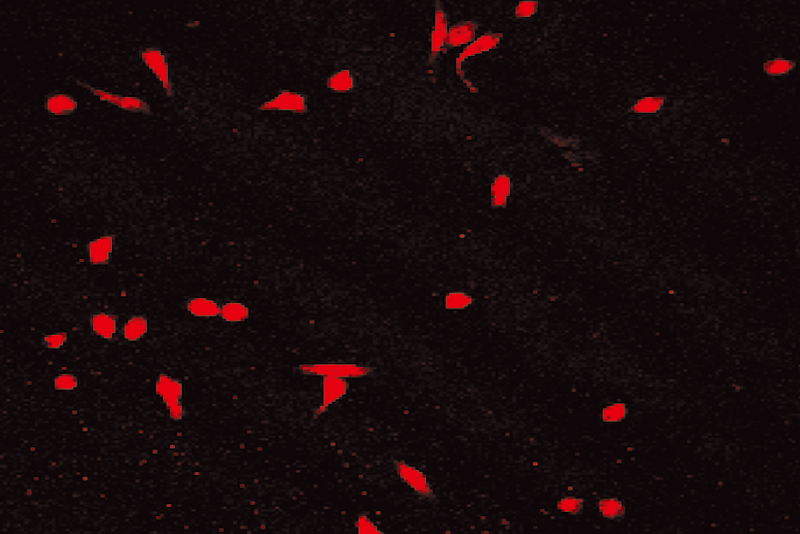
Fig. 1. Cobblestone-like EPC (6th day). Fig. 2. Positive for DiI-Ac-LDL.
Fig. 3. Positive for FITC-lectin-UEA-1. Fig. 4. Double positive for DiI-Ac-LDL and FITC-lectin-UEA-1.
Discussion
Bone marrow (BM) has been reported to be the primary site of origin of progenitor cells.[1,5,6] However, other hematopoietic and nonhematopoietic tissues also contribute to the circulating progenitor cell pool. EPCs from peripheral blood are maturer than the premature bone marrow-derived cells. However, the latter have a much greater potential to differentiate into mature ECs. Recently, Masuda et al[7] reported that EPCs can be isolated from human umbilical cord blood (HUCB) and fetal liver. Compared with BM and HUCB, peripheral blood samples are easier to be obtained, avoiding ethnical consideration of heterogeneous marrow and umbilical cord blood. Meanwhile, individuals suffer much less from blood collection than by other means.
Inevitably, such a small number of EPCs exists as a considerate limit. Researchers have estimated that the number of circulating EPCs is about 500-1000 per milliliter.[8] Fortunately, mobilization and other procedures of expansion are available for obtaining enough therapeutic EPCs.[9] In the past decade, accumulating evidences have demonstrated that circulating EPCs will dramatically increase with such stimulations as limb ischemia and ischemic heart disease, while playing an important role in postnatal vascularization,[10,11] in addition to mobilization of EPCs by cytokine supplement.[3,6,12-14] Kocher et al[12] reported that stimulation with G-CSF increases the number of CD34-positive cells expressing endothelial markers. G-CSF will also enhance the endothelialization of small-caliber prosthetic grafts in association with an elevation of circulating EPCs.
In the present study, rhG-CSF was used to mobilize more EPCs into peripheral blood. Compared with others, an equivalent single volume of rhG-CSF was administered, but the gross quantity was relatively less. It has been recognized that G-CSF can mobilize progenitor cells into peripheral blood by inhibiting the expression of certain adhesion molecules, while decreasing adhesion of progenitor cells with marrow stroma. As a result, prominent mobilization of EPCs was seen.
The most frequently used hematopoietic growth factors for peripheral blood stem cell mobilization are G-CSF and GM-CSF. The expression of the receptors of G-CSF and GM-CSF is very low on the more primitive progenitor cells and increases during myeloid or myelomonocytic differentiation.[15,16] The G-CSF receptor is also expressed on stromal cells of the BM. The mobilizing mechanism may be partly due to the action of G-CSF on BM stroma.[15] In this process, matrix metalloproteinase plays an important role.
Nowadays, many researchers have been using microbeads or flow cytometry to sort out CD34+ cells and their subtypes,[17] and to get almost fully purified cells. But these techniques are not the best for sorting a relatively large numer of EPCs because of its high expense and the complicated procedures. Thus, we isolated MNCs as a rich source of EPCs from peripheral blood by Ficoll density gradient centrifugation.
So far, exact phenotypes of EPCs remain unknown.[18] It is still unrealistic to isolate EPCs by specific molecular markers on cell surface. In our study, the expression of CD31, CD34, vWF and Flk-1 varied, continuously indicating that EPCs can not be identified with the solid immunocytochemistry system.
In the present study, we can identify EPCs by its origin and differentiation features. We investigated the differentiation inducer of the endothelial-like cells and observed that adherent and cobble-like cells overexpressed vWF, CD31 and Flk-1, specific for EPCs.[19] Although different surface markers are used in EPC isolation, it has been accepted that CD34 and Flk-1 are specific and characterized for EPC. In addition, the EPCs demonstrated double-positive fluorescence for LDL and lectin-UEA-1, which are specific for endothelial phenotype. EPCs and hematopoietic cells have the same precursor, which provides them with so many common surface markers,[20] so do EPCs and endothelial cells. However, transfusion of mature endothelial cells can not repair vascular injury from severe tissue ischemia.[14,21] Unlike endothelial cells, EPCs contribute to neovascularization and have a delayed increment potential, 50-fold of the former.[22] Another problem is that some adult stem-cells also express CD34, CD31, Tie-1, Tie-2 or vWF,[23] which greatly burdens the obtaining of purified EPCs. In other words, the isolated EPCs in our study are a mixture of a group of stem and progenitor cells in different developing stages, although the majority of them are EPCs.
VEGF and bFGF are used to induce transdifferen-tiation of MNCs to EPCs and preplated fibronectin worked as adherence wall of EPCs. Under this condition, 3 days after planting cells in specific culture media, few erythrocytes may sediment on the base under a inverted phase contrast microscope. Repeated washing can eliminate almost all the unwanted cells for complete purification of EPCs.
The present study demonstrated that relatively purified EPCs can be obtained from peripheral blood after a series of procedures, which is of great significance for the future application in KD therapy and other angiogenic fields.
Funding: This work was supported by a grant from the National Natural Science Foundation of China (No. 30271294).
Ethical approval: Not needed.
Competing interest: No benefit in any form have been reveived or will be reveived from any commercial party related directly or indirectly to the subjects of this article.
Contributors: PSL wrote the first draft of this paper. All authors contributed to the intellectual content and approved the final version.
References
1 Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative endothelial progenitor cells for angiogenesis. Science 1997;275:964-967.
2 Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Tokutomi T, Sekine I. Circulating endothelial cells in Kawasaki disease. Clin Exp Immunol 2003;131:536-540.
3 Kalka C, Masuda H, Takahashi T, Gordan R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor (165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 2000;86:1198-1202.
4 Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Aldler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 2001;108:391-397.
5 Kaushal S, Amiel GE, Gulserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 2001;7:1035-1040.
6 Bhattacharya V, Shi Q, Ishida A, Sauvage LR, Hammond WP, Wu MH, et al. Administration of granulocyte colony-stimulating factor enhances endothelialization and microvessel formation in small caliber synthetic vascular grafts. J Vasc Surg 2000;32:116-123.
7 Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res 2003;58:390-398.
8 Wang WD, Chen ZT. Evolution of endothelial progenitor cell research. J Chin Microcir 2002;6:6-8.
9 Asahara T, Murohara T, Sullivan A, Silver M, Van der zee R, Schatteman G, et al. Isolation of putative endothelial cells for angiogenesis. Science 1997;275:964-967.
10 Laufs U, Werner N, Link A, Endres M, Wassamann S, Jurgens K, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004;109:220-226.
11 Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke 2002;33:1362-1368.
12 Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430-436.
13 Shi Q, Bhattacharya V, Hong-De Wu M, Sauvage LR. Utilizing granulocyte colonystimulating factor to enhance vascular graft endothelialization from circulating blood cells. Ann Vasc Surg 2002;16:314-320.
14 Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex-vivo expandedendothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000;97:3422-3427.
15 Takahashi T, Kalka C, Masuda H, Chen D, Siver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999;4:434-438.
16 Lund-Johansen F, Houck D, Hoffman R, Davis K, Olveus J. Primitive human hematopoietic progenitor cells express receptors for granulocyte-macrophage colony-stimulating factor. Exp Hematol 1999;27:762-772.
17 Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood 2000;95:3025-3031.
18 Matsumoto K, Yasui K, Yamashita N, Horie Y, Yamada T, Tani Y, et al. In vitro proliferation potential of AC133 positive cells in peripheral blood. Stem cells 2000;18:196-203.
19 Kong D, Melo LG, Mangi AA, Zhang L, Lopez I, Perrela MA, et al. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation 2004;110:2039-2046.
20 Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development 1998;125:725-732.
21 Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004;24:288-293.
22 Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 2004;287:C572-579.
23 Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000;105:1527-1536.
Received May 5, 2006 Accepted after revision August 30, 2006

