|
Differentiation of neuroblastoma cells induced
by nerve growth factor gene transfection
Qian Dong, Qiang Gao, Hong-Ting Lu, Li-Rong Sun, Lin Hou, Yu Cheng
Qingdao, China
Author Affiliations: Department of Pediatric Surgery, Affiliated Hospital of Medical College, Qingdao University, Qingdao 266003, (Dong Q, Lu HT, Sun LR, Hou L, Cheng Y); Department of Pediatric Surgery, Qingdao Children's Hospital, Qingdao 266011, China (Gao Q)
Corresponding Author: Qian Dong, MD, Department of Pediatric Surgery, Affiliated Hospital of Medical College, Qingdao University, Qingdao 266003, China (Tel: 86-532-82911885; Fax: 86-532-82911999; Email: dong.qian@tom.com)
Background: Neuroblastoma (NB) is a very common childhood malignant tumor with considerable morbidity and mortality. Nevertheless, some cases can disappear spontaneously and be induced to differentiate into mature cells in vitro. It may be a potential treatment method to induce NB cells to differentiate into mature cells. This study was undertaken to observe the differentiation of neuroblastoma cell line transfected with nerve growth factor (NGF) gene and investigate the role of NGF in the differentiation.
Methods: Tumor specimens of NB patients were collected for primary cell culture and the cells were separated and purified to be cell line as a cell model. The plasmid containing NGF gene was transfected into NB cells mediated by liposome. Morphological changes were observed under a phase-contrast microscope. Cell proliferation was determined by the MTT method and mitosis index.
Results: The expression of NGF was higher after gene transfection, the cell proliferation was inhibited and morphological changes occurred. Total RNA separated by agrose gel electrophoresis resulted in clear 28s and 18s, indicating that RNA quality was good. RT-PCR products about 726 bp, matching NGF size, indicated that NB cells can express a certain amount of NGF, and the expression was increased after NGF gene transfection. Forty-eight hours after NGF gene transfection, morphodifferentiation presented like neurodendrite and axons, which were similar to mature ganglion cells.
Conclusions: The established NB cell line is an induced type (type N). The transgenic NB cells can highly express NGF, which can inhibit proliferation and induce differentiation.
Key words: NGF; transfection; neuroblastoma; cell line; differentiation
World J Pediatr 2007;3(2):115-120
Introduction
Neuroblastoma (NB) originated from the primitive neural crest is one of the most common solid tumors of childhood.[1] Metastasis may occur at the early stage of NB, but in some cases the tumor can disappear spontaneously and be differentiated into mature cells in vitro. How to induce NB cells to differentiate into mature cells in a man-controlled manner is a focus of interest in the tumor study.[2-4] Nerve growth factor (NGF) plays an important role in the development, growth, repair and differentiation of nerve cells, and is also one of the mitogenic factors with strong actions. The factor has extensive effects and considerable effector cells. This study was undertaken to observe effects of NGF on differentiation of NB cells by transfecting the NGF gene into NB cells and explore the underlying mechanisms, thus providing a theoretical foundation for the transgenic treatment of NB.
Methods
Reagents
DMEM was purchased from Gibco-BRL Company; pcDNA4/HisMax plasmid containing the NGF gene was kindly provided by Dr. Lu Zhenghua; pcDNA4/HisMax was purchased from Invitrogen Company; RT-PCR and liposome transfection kits were from QIAGEN Company; agents for internal control of RT-PCR were from SBS Biotech Company; 2% orcein acetate solution was prepared in the laboratory.
Neuroblastoma primary cell culture
Primary cells were isolated from the surgical sample of a boy suffering from neuroblastoma with bone metastasis.[5] The cells were pathologically confirmed in Evans stage IV, maintained in DMEM containing 10% FBS, 100 U/ml penicillin and streptomycin at 37ºC in a 5% CO2 incubator, and passaged 3 times for experiments.[6]
Liposome transfection
The NGF gene was transfected into NB cells using Effectene (transfection reagent selector kit) according to the manufacturer's instructions. Briefly, 2-8 ¡Á105 cells were seeded in a 60 mm culture plate and cultured at 37ºC in a 5% CO2 incubator overnight. One ¦Ìg DNA was diluted with buffer to a minimum concentration of 0.1 ¦Ìg/¦Ìl, then added with buffer EC to a final volume of 150 ¦Ìl, followed by adding 8 ¦Ìl Enhencer and mixing. The mixture was incubated at room temperature for 5 minutes followed by adding 25 ¦Ìl of liposome, mixing up-side-down and incubating at room temperature for 10 minutes. Finally a compound was formed.
The medium in the culture plate was discarded and washed with PBS. Four ml of fresh medium was mixed with the DNA compound, then 200 ¦Ìl of the mixture was added into each well.
RT-PCR
Total RNA was extracted using Trizol reagent (Gibico) according to the manufacturer's instructions, dissolved in diethylpyrocarbonate-treated water, and quantitated with a spectrophotometer. The quantity of RNA was determined by agarose electrophoresis. The 50 ¦Ìl one-step RT-PCR reaction system contained 10 ¦Ìl of 5 ¡Á QINGEN one-step RT-PCR buffer, 2 ¦Ìl of d NTP MIX, 1 ¦Ìl of 60 ¦Ìm primers, 12 ¦Ìl of Rnase-free water, and 2 ¦Ìl of QIAGEN one-step RT-PCR enzyme MIX. The PCR condition was as follows: reverse transcription at 50ºC for 30 minutes, then 38 cycles at 94ºC for 1 minute, 56ºC for 1 minute, and 72ºC for 1 minute. The sequences of NGF primers used were as follows: sense: 5'ACC CCC ACT GAA AAA GAT GA3' and antisense: 5'ATC TTC AAA CCT CCA TGA TG3'. The ¦Â-actin was amplified as an internal control as reported previously. The 726 bp PCR products were separated by electrophoresis in a 1% agarose gel and stained with SYBRÒ Green I. The target bands were analyzed densitometrically by using the Vistra Fluor Imager SI (Molecular Dynamics Inc., USA).
Cell groups
NB cells were divided into four groups: NB cells untreated (A); NB cells treated with liposome only (B); NB cells transfected with blank vectors (C); and NB cells transfected with the NGF gene (D).
Morphological analysis
1 ¡Á 103/cm3 cells from the four groups were seeded in 25 ml flasks. According to the Sandquist method[7], the cells with length of one or more than one axon exceeding double diameter of cell body were regarded as differentiated cells. The differentiated cells were observed under a phase-contrast microscope. 200 cells were counted in five visual fields and the percentage was calculated.
In vitro cell growth assay
Proliferation of NB cells was assayed using the MTT method.[7] Approximately 5000 cells of each well from each group were plated in a 96-well plate and incubated for 4 hours in 100 ¦Ìl of cultured medium. The medium was discarded and the cells were washed with PBS. 20 ¦Ìl of MTT was added to each well at a concentration of 5 mg/ml. After incubation at 37ºC for 4 hours in an atmosphere of 5% CO2, the MTT was removed and replaced with 150 ¦Ìl of DMSO by shaking for 10 minutes till crystals were dissolved. The optical density (OD) value of each well was measured using a microculture plate reader with a test wavelength of 525 nm.
Detection of NGF protein expression level by immunohistochemistry
Serum protein (SP) staining was used to detect the expression of NGF in NB. This staining is a standard immunohistochemical method for detecting protein.
Mitosis index assay
Forty-eight hours after gene transfection, 2-5 ¡Á 104 cells/well from each group were plated in a 6-well plate and incubated for 24 hours. The plates were then centrifuged at 1500 rmp for 10 minutes, and as a result, round cells in mitosis stage combined tightly to the plate. The culture medium was replaced with 1.9 ml of 0.684% citric acid, trisodium salt, and 0.4 ml distilled water. The plate was incubated at room temperature (RT) for 10 minutes, and rinsed subsequently with 2.3 ml of fixing solution (ethanol/glacial acetic acid, 3:1). Then the plate was added with 2 ml fixing solution for further incubation for 10 minutes, followed by discarding and natural drying of the solution. The cells were stained with filtrated orcein acetate solution for 10 minutes, followed by washing with pure ethanol and drying naturally. The nuclei of mitosis were observed under a low-power light microscope. Five visual fields were counted in each well and 200 cells were counted in each visual field. The percentage of cells with mitosis was calculated.
Statistical analysis
A statistical package SPSS10.0 (SPSS incorporated, Chicago, USA) was used for all analyses. ANOVA was used to assess the significance of differences among the four groups. A value of P<0.05 was considered statistically significant.
Results
Detection of NGF in NGF gene-transfected NB cells
Total RNA of cells in the four groups was extracted and then subjected to electrophoresis. The RNA bands of 28s, 18s and 5s in running gel were noted in the four groups, demonstrating that extractives were total RNA (Fig. 1).
RT-PCR showed a DNA band at the expected site (726 bp), which was identical in size to the expected amplified products of NGF. In groups A, B and C, expression of the NGF gene was equal in amount but in group D it was greater. This finding suggests that NB cells themselves can express a certain amount of NGF, and can increase this expression after transgenic treatment (Fig. 2, Table 1).
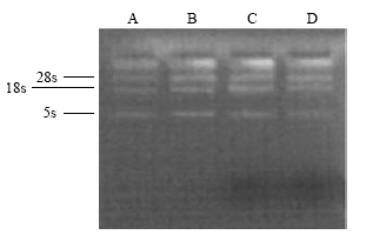 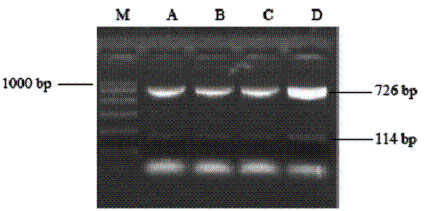
Fig.1 Fig.2
Fig. 1. Electrophoresis image of total RNA extracted. Fig. 2. Electrophoresis image of RT-PCR products in the four groups.
Table 1. Semi-quantitative analysis of RT-PCR results
|
Group |
Values after semiquantitative analysis |
|
A |
B |
C |
D |
|
NGF DNA (726 bp) |
248 |
237 |
233 |
335 |
|
¦Â-actin (114 bp) |
207 |
205 |
207 |
204 |
|
Ratio |
1.20 |
1.16 |
1.13 |
1.64* |
*: Compared with the other three groups, values of group D were statistically different (P<0.01).
SP staining of NB cells
Since the NB cells with the transfected NGF gene stained deeper than those without the transfected NGF gene, the expression of NGF was higher in NB cells with the transfected NGF gene (Figs. 3, 4).
Inhibition of NB cell line proliferation by NGF
After NGF gene transfection, NB cells reduced the ability to proliferate. Comparison of groups A, B and C showed no significant changes in proliferation of NB cells (Tables 2, 3).
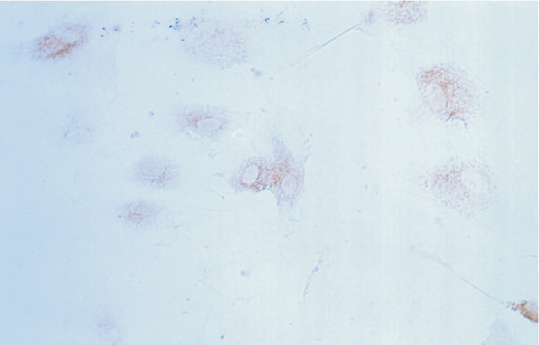 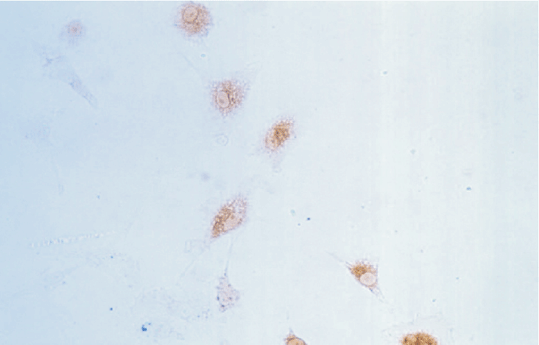
Fig. 3 Fig. 4
Fig. 3. SP staining of normal NB cells. Fig. 4. SP staining of NB cells with the transfected NGF gene.
Table 2. The results of MTT assay (means ¡À SD)
|
Group |
Optical density value of the four groups |
|
A (n=6) |
0.451¡À0.030 |
|
B (n=6) |
0.441¡À0.029 |
|
C (n=6) |
0.438¡À0.007 |
|
D (n=6) |
0.373¡À0.024* |
*: Comparison of groups A, B, C and D showed significantly different changes in NB cells (P<0.01), but no significant difference among groups A, B and C.
Table 3. Percentage of mitotic cells (means ¡À SD)
|
Group |
Percentage of mitotic cells in the four groups (%) |
|
A (n=1000) |
6.733¡À0.403 |
|
B (n=1000) |
6.600¡À0.219 |
|
C (n=1000) |
6.483¡À0.371 |
|
D (n=1000) |
4.383¡À0.248* |
*: Five visual fields were counted in each well and 200 cells were counted in each visual field, so that 1000 cells of each group were counted and the percentage of cells with mitosis was calculated. Compared with groups A, B and C, group D showed significant difference in changes of NB (P<0.01), but no significant difference was found among groups A, B and C.
Effect of NGF on differentiation of NB cells
NB cells were little shuttle-shaped, but some appeared teardrop-shaped, and a minority were triangular (Fig. 5). Forty-eight hours after NGF gene transfection, morphodifferentiation presented nerve process forma-tion, like neurodendrite and axons, which were similar to mature ganglion cells (Fig. 6). Cells in the four groups were observed at multiple time points and the differentiation percentages calculated are shown in Table 4. In groups A, B and D, the percentages of differentiated cells were not significantly different at various time points, but those of transgenic cells in group D were markedly increased at 2 days after transfection and significantly different from those in group A (P<0.05). With time prolonging, the percentage was gradually increased (Figs. 7-10).
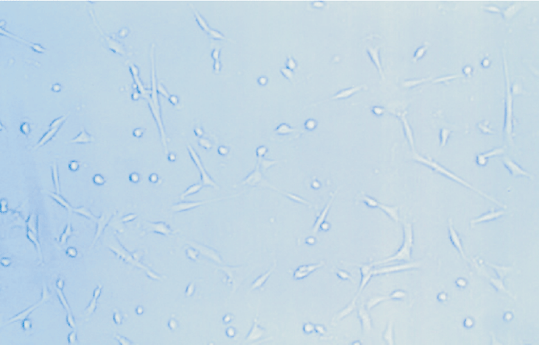 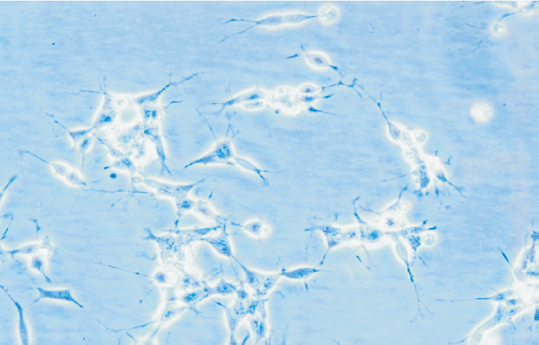 
Fig. 5 Fig. 6 Fig. 7
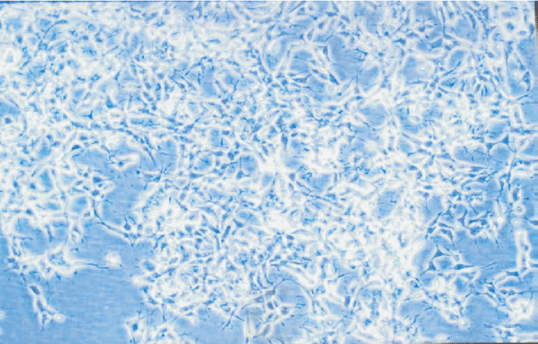 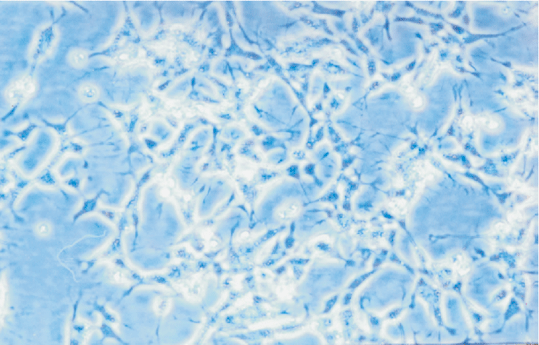 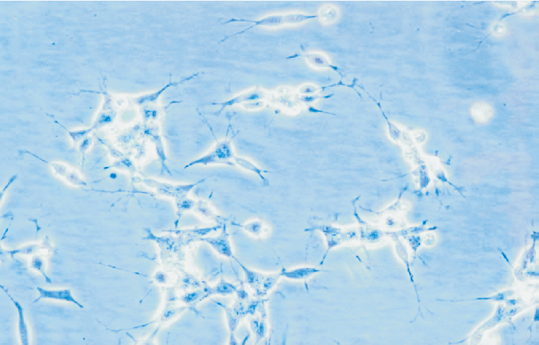
Fig. 8 Fig. 9 Fig.10
Fig. 5. Morphology of NB cells prior to transgenic treatment (100 ¡Á original magnification).Fig. 6 Morphology of NB cells after transgenic treatment (100 ¡Á original magnification).Fig. 7 Morphology of NB cells at 0 day after transgenic treatment (40 ¡Á original magnification).Fig. 8 Morphology of NB cells at 2 days after transgenic treatment (40 ¡Á original magnification). Fig. 9 Morphology of NB cells at 4 days after transgenic treatment (100 ¡Á original magnification). Fig. 10 Morphology of NB cells at 8 days after transgenic treatment (100 ¡Á original magnification).
Table 4. Percentage of differentiated cells (means ¡À SD)
|
Time point (d) |
Percentage of differentiated cells in the four groups (%) |
|
A |
B |
C |
D |
|
0 |
5.50¡À0.50 |
5.80¡À0.57 |
6.20¡À0.27 |
6.60¡À0.30 |
|
2 |
7.10¡À0.42 |
6.40¡À0.40 |
6.90¡À0.51 |
15.90¡À0.76* |
|
4 |
8.20¡À0.57 |
8.10¡À0.35 |
7.80¡À0.64 |
33.10¡À1.30* |
|
6 |
8.50¡À0.50 |
8.40¡À0.58 |
8.30¡À0.71 |
35.20¡À0.74* |
|
8 |
8.90¡À0.74 |
9.10¡À0.64 |
8.50¡À0.42 |
57.50¡À2.13* |
*: Compared with groups A, B and C, group D showed significant difference in changes of NB cells (P<0.01), but no significant difference among groups A, B and C.
Discussion
The establishment of NB cell line is essential to the study of transgenic induction of NB differentiation and to further study of the relationship between biological characteristics of NB and its malignant degree, staging, prognosis and auto-disappearance.
Nerve growth factor is one of the early discovered growth factors, named as ¦Â-NGF because the ¦Â subunit is its active part. This factor, a relatively potent mitogenic factor, has extensive effects and considerable effector cells. It plays roles in the survival, growth, development, differentiation, damage-repair and regeneration of neurons.[8] In this study, we established a NB cell line, and then transfected the NGF gene into NB cells by the liposome-mediated method. We found that the cells could highly express the NGF gene and reduce their capabilities of mitosis and propagation, and the morphodifferentiation could take place, demonstrating that the established cell line is a cell type that can differentiate. The differentiation of NB cells that can highly express NGF, we think, is mediated by NGF receptors. High expression of the NGF gene can produce NGF in a great amount, leading to an increase in binding of NGF to its receptor and as a result, an elevation in inducing differentiation and inhibiting propagation.
It is generally believed that mainly depending on the involvement of TrkA, one of its high-affinity receptors, NGF is able to promote NB cell differentiation. TrkA mainly comprises gp140trk, a transmembrane tyrosine kinase and a product of proto-oncogene Trk. Binding of NGF to TrkA can affect transcription and translation of multiple genes by activating the RAS/MAPK signal pathway, manifesting a modulation on cell growth.[9] Interaction of NGF and TrkA is an important factor that affects the growth and progression of malignant cells, yet binding of TrkA and its ligand, NGF, triggers cascade reactions of signal pathways, or induces in vitro NB cells to differentiate into neurons and also plays an important role in the course of differentiation of in vivo NB cells into mature cells.[10] Recent studies[11,12] have revealed that TrkA-mediated signal pathways play pivotal roles in NGF-induced differentiation of many in vitro cultured cells derived from nerve tissue, such as NB. Activation of the RAS/MAPK signal pathway may be the most important for NGF-induced differentiation of NB cells. A study demonstrated that NGF and the c-myc gene, an apoptosis-associated gene, can jointly regulate cell apoptosis, and NGF can selectively induce NB to differentiate into neurons.[9] In addition, c-myc expression is decreased in differentiated NB cells induced by NGF.[9] These data have indicated that probably via inhibiting oncogene c-myc expression, NGF may take its effect in promoting cell differentiation. Chen and his colleagues[10] thought that down-regulation of c-myc expression is one of the important events occurring during the course of NGF-induced NB differentiation.
As far as favorable differentiation of malignances into mature cells is concerned, this study provides an important indicator for experimental transgenic treatment of NB. The detailed underlying mechanism of modulation in differentiation of NB cells by the highly-expressed NGF gene awaits further study.
Funding: This study was supported by a grant from the National Natural Science Foundation of China (No. 30170974, 30471800).
Ethical approval: Not needed.
Competing interest: No benefits in any form have been received or will be received from any commercial party related directly or indirectly to the subject of this article.
Contributors: DQ and GQ wrote the first draft of this paper. All authors contributed to the intellectual content and approved the final version. DQ is the guarantor.
References
1 Redlinger RE Jr, Mailliard RB, Barksdale EM Jr. Neuroblastoma and dendritic cell function. Semin Pediatr Surg 2004;13:61-71.
2 Bogenmann E, Peterson S, Maekawa K, Matsushima H. Regulation of NGF responsiveness in human neuroblastoma. Oncogene 1998;17:2367-2376.
3 Lu HT, Dong Q, Yang CM, Luo B, Sun LR. Establishment of neuroblastoma (NB) cell line and induction of cell differentiation with all-trans retinoic acid. Chin J Pediatr Surg 2004;25:71-74.
4 Cheng Y, Dong Q, Sun LR, Yang CM, Jiang BX. Correlation between expression of MMP-2, MMP-9, TIMP-2, TIMP-1and metastasis of neuroblastoma. Chin J Oncol 2005;27:164-166.
5 Gao Q, Dong Q, Lu HT, Luo B, Lu ZH, Sun LR. Establishment of neuroblastoma cell line in vitro. Shandong Med J 2004;44:5-6.
6 Shitu ZQ, Wu JZ. Cell Culture, 1st ed. Xi'an: World Publishing Co. Ltd, 1999: 71-72.
7 Woo CW, Lucarelli E, Thiele CJ. NGF activation of TrkA decreases N-myc expression via MAPK path leading to a decrease in neuroblastoma cell number. Oncogene 2004;23: 1522-1530.
8 Sun WM. The technology of studying cytokines, 1st ed. Beijing: People's Medical Publishing House, 1999: 65-66, 82-83, 695-700.
9 Hao J, Gao YM, Liu K. Advances in NGF study. Prog Anat Sci 1999;5:203-207.
10 Chen J, Chen TH, Ross AH. Changes of oncogenes expression in nerve growth factor-induced differentiation of neuroblastoma cells. Chin J Pathol 1991;20:7-9.
11 Oe T, Sasayama T, Nagashima T, Muramoto M, Yamazaki T, Morikawa N, et al. Differences in gene expression profile among SH-SY5Y neuroblastoma subclones with different neurite outgrowth responses to nerve growth factor. J Neurochem 2005;94:1264-1276.
12 Schramm A, Schulte JH, Astrahantseff K, Apostolov O, Limpt V, Sieverts H, et al. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett 2005;228:143-153.
Received July 13, 2006; Accepted after revision April 1, 2007
|

