|
Expression of Fas and FasL in human
neuroblastoma and its clinical significance
Qiang-Song Tong, Li-Duan Zheng, Shao-Tao Tang, Shi-Wang Li, Guo-Song Jiang, Jia-Bin Cai, Yuan Liu, Qing-Lan Ruan
Wuhan, China
Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China (Tong QS, Tang ST, Li SW, Jiang GS, Cai JB, Liu Y, Ruan QL); Department of Pathology, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China (Zheng LD)
Corresponding Author: Qiang-Song Tong, PhD, MD, Department of Pediatric Surgery, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China (Tel: 86-27-85991567; Email: qstong@mails.tjmu.edu.cn)
Background: Recent evidences indicated that tumor cells could induce apoptosis of T cells by combining Fas on active T cells' surface with self-expressed FasL to escape immune surveillance. This study was undertaken to detect the expression of the Fas/FasL system in neuroblastoma (NB) tissues and its clinical significance in clinical practice.
Methods: Immunohistochemical staining was performed to detect the expression of Fas and FasL in 40 cases of NB. The relations among Fas and FasL expression, clinical stages, pathological types, and postoperative survival time of patients were analyzed.
Results: The positive expression rates of Fas in I-II, III-IV and IV-S stages were 64.3%, 22.2% and 50.0% respectively (P<0.05). The positive expression rate of FasL in III-IV stage was 33.3%, which was significantly higher than that in I-II (21.4%) and IV-S stages (12.5%). FasL expression rates in patients with NB (28.1%) and unfavorable histology (UFH) (33.3%) were higher than those of patients with ganglioneuroblastoma (GNB) (12.5%) and favorable histology (FH) (15.8%). However, Fas expression rates in patients with GNB (50%) and FH (63.2%) were higher than those of patients with NB (40.6%) and UFH (23.8%). A positive correlation was observed between Fas expression and postoperative survival time (n=25, r=0.354), but no obvious correlation between FasL expression and postoperative survival time.
Conclusions: Abnormalities of the Fas/FasL system are related to the development of NB. Detection of the expression of Fas/FasL system may be useful to evaluate the clinical prognosis of NB.
Key words: neuroblastoma; Fas; FasL; gene expression; immune escape
World J Pediatr 2007;3(3):209-213
Introduction
Neuroblastoma (NB) is the most common malignant solid neoplasm originating from the sympathetic nerve system in childhood. Recent studies have indicated that immune escape of cancer cells plays an important role in the development of neoplasms. As an important factor for cellular apoptosis, the Fas/Fas ligand (FasL) system participates in the immune escape of tumor tissues.[1] In this study, we determined the expression of Fas and FasL in NB by an immunohistochemical method to explore the relationship between immune escape and NB.
Methods
Tissue samples
The tissue samples from 40 pediatric patients with NB were obtained surgically during 1997-2006 at the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan. The age of the patients ranged from 4 months to 7 years (average, 2 years and 5 months). In this series, 18 were male and 22 female. According to the Evans Classification of Clinical Stages,[2] 6 patients belonged to stage I, 8 stage II, 8 stage III, 10 stage IV, and 8 stage IV-S. According to the method of Shimada et al,[3] the mitosis karyorrhexis index (MKI) and pathological types were evaluated. As a result, 19 patients had favorable histology (FH) and 21 unfavorable histology (UFH). The case numbers for ganglioneuroblastoma (GNB) and NB were 8 and 32 respectively. No patient was given radiotherapy or chemotherapy before operation. Twenty-five patients were followed up for 6 months to 5 years after operation.
Reagents
Mouse monoclonal antibodies (mAb) for Fas and FasL were purchased from Santa Cruz Company, USA. Other reagents were purchased from Beijing ZhongSan Biotechnology Company, China.
Immunohistochemical staining
Tissue samples were fixed with 10% formalin, embedded in paraffin, and sliced into 4-5 mm flakes. The streptavdin-peroxidase-biotin (SP) immunohistochemical method was used to detect the expression of Fas and FasL. Briefly, after the splices were dewaxed and hydrated, antigens were prepared using the bathing method. When citric acid buffer (pH 6.0) was heated up to 95ºC-99ºC, the slices were bathed in it for 30 minutes and then cooled at room temperature for 20 minutes. The slices were taken out and washed with phosphate buffered saline. 3% H2O2 was used to block intrinsic peroxidase activities of the prepared slices. After being incubated with normal serum, mAbs for Fas and FasL (working concentration being 1:200 and 1:400 respectively) were incubated with the slices at 4ºC overnight. The second antibody was from SP reagent kit. After being stained with diaminobenzidine (DAB), the slices were stained with hematoxylin. Instead of the first antibody, rabbit serum was applied in the negative control slices.
Determination of staining results
Under the light microscope, Fas and FasL positive immunological staining reactions presented brown-yellow granules in the cytoplasm and/or cell membrane. Five to ten high multiple microscope fields were randomly selected to calculate the numbers of positive staining cells in 1000 tumor cells by HPIAS-1000 (High Resolution Pathological Image & Word Analysis System, Bejing, China).[4] Positive index (PI) = (number of positive staining cells/1000) ¡Á 100%. The staining results were determined with the following criteria: when PI was less than 5%, it was considered as negative; when PI was between 6% and 25%, it was considered as weak positive; when PI was between 26% and 50%, it was considered as moderate positive; when PI was more than 50%, it was considered as strong positive.
Statistical analysis
The SPSS 12.0 statistical software was used for data analysis. The significances between categorical variables were analyzed using Fisher's exact test. The correlation between positive expression rates of Fas/FasL and patients' postoperative survival time was also analyzed. P values less than 0.05 were considered statistically significant.
Results
Correlation of Fas/FasL expression with clinical stages of NB
As shown in Table 1 and Fig. A-F, 64.3% (9/14) NB samples of I-II stage patients had dispersed or local Fas expression, 22.2% (4/18) NB samples of III-IV stage were positive for Fas expression, compared with that (50.0%, 4/8) of IV-S stage. The difference of Fas expression in various stages was significant (P<0.05). FasL positive expression rates in NB samples of III-IV and IV-S stage patients were 33.3% (6/18) and 12.5% (1/8) respectively. Their difference was significant (P<0.05). Almost 21.4% (3/14) NB samples of I-II stage patients expressed FasL, mostly localizing in a few tumor cells as focus. Compared with those of III-IV and IV-S stages, the difference in FasL expression was significant (P<0.01).
Correlation of Fas/FasL expression with pathological types of NB
The positive expression rates of Fas and FasL in GNB were 50% (4/8) and 12.5% (1/8) respectively, compared with those [40.6% (13/32) and 28.1% (9/32) respectively] in NB. The positive expression rates of Fas and FasL in UFH were 23.8% (5/21) and 33.3% (7/21), compared with those [63.2% (12/19) and 15.8% (3/19)] in FH. When groups were classified as GNB and NB, and FH and UFH, the Fisher's exact test was performed. The positive rates of FasL in NB and UFH were higher than those in GNB and FH; however, the positive expression rates of Fas in GNB and FH were higher than those in NB and UFH (Table 2).
Table 1. Fas/FasL expression in various clinical stages of neuroblastoma
|
Clinical stage of neuroblastoma |
Fas
|
FasL
|
|
I-II |
64.3% (9/14) |
21.4% (3/14) |
|
III-IV |
22.2% (4/18) |
33.3% (6/18) |
|
IV-S |
50.0% (4/8) |
12.5% (1/8) |
Table 2. Fas/FasL expression of various pathological types of NB
|
|
Fas expression |
P value |
FasL expression |
P value |
|
Positive |
Negative |
Positive |
Negative |
|
Histological types |
|
|
|
|
|
|
|
NB |
13 |
19 |
0.005 |
9 |
23 |
0.024 |
|
GNB |
4 |
4 |
1 |
7 |
|
Shimada classification |
|
|
|
|
|
|
|
FH |
12 |
7 |
0.012 |
3 |
16 |
0.018 |
|
UFH |
5 |
16 |
7 |
14 |
NB: neuroblastoma; GNB: ganglioneuroblastoma; FH: favorable histology; UFH: unfavorable histology.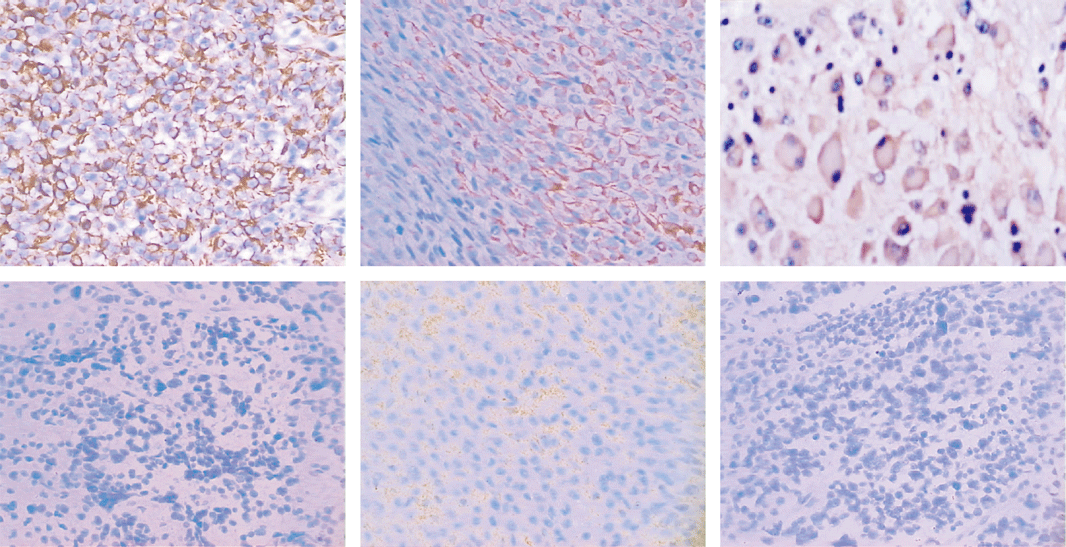
Fig. Expression of Fas and FasL in NB tissues. A: Fas strongly positive expression in I stage NB tissues (original magnification ¡Á 200). B: Fas locally positive expression in II stage NB tissues (original magnification ¡Á 200). C: Fas strongly positive expression in ganglioneuroblastoma tissues (original magnification ¡Á 400). D: Fas negative expression in IV stage NB tissues (original magnification ¡Á 200). E: FasL strongly positive expression in III stage NB tissues (original magnification ¡Á 200). F: FasL negative expression in II stage NB tissues (original magnification ¡Á 200). NB: neuroblastoma.
Correlation of Fas/FasL expression with postoperative survival of NB
The median survival time and accumulative total survival rate in Fas positive patients of 2 years old (25 months, 65.7%) were longer than those in Fas negative patients (17 months, 33.6%) (P<0.01). There was a positive correlation between Fas and survival time (n=25, r=0.354). The median survival time and accumulative total survival rate in FasL positive patients of 2 years old (15 months, 35.7%) were shorter than those in FasL negative patients (23 months, 44.8%) (P=0.09).
Discussion
Neuroblastoma is known for its clinical and biological heterogeneity. Although some genetic and biological features, such as 1p chromosome deletions,[5] N-myc,[6] TRKA,[7] TRKB,[7] and CD44[8] are believed to account for its particular behavior, the role of immune surveillance in NB is poorly understood. According to some recent reports, NB cells could escape cytotoxic T-lymphocyte recognition by down-regulating major histocompatibility complex (MHC) antigen expression, but they are still highly susceptible to natural killer (NK) and lymphokine-activated killer (LAK) cell cytotoxicity.[9] The Fas/FasL system is a brand new mechanism for tumor cells to escape the immune attacks of different effector cells.[10] Fas (APO-1, CD95) is a transmembrane protein transducing cellular apoptosis signals, mostly being expressed in the immature lymphocytes, ovary and heart.[10] Fas ligand (FasL) is mostly expressed in active effector cells such as NK and cytotoxic T-lymphocyte (CTL).[11] When Fas protein of target cells is combined with FasL of effector cells, cellular apoptosis is induced by caspase cascades, which has been shown to be critical to the maintenance of immunological homeostasis and peripheral tolerance by deletion of activated T-lymphocytes.[12]
Tumor cells could escape immune surveillance through the Fas/FasL pathway. To escape the immune surveillance, some non-lymphatic tumor cells have been shown to express FasL that induces apoptosis of tumor-infiltrating lymphocyte (TIL) bearing Fas.[13] Many types of tumors such as melanoma, lung cancer, colon cancer, liver cancer and glioma expressed high levels of FasL.[14] It was also illuminated that 16 kinds of lung cancer cell lines expressed FasL by immunohistochemical and reverse transcription-polymerase chain reaction (RT-PCR) methods.[15] Using the same methods, it was also found that 16 of 61 lung cancer patients were positive for FasL expression.[16] Gratas et al[17] studied the expression of FasL in esophageal cancer cell lines and clinical samples, and found that FasL expressions were almost enhanced in all samples.
Besides, tumor cells could express non-functional Fas, down-regulate or even express no Fas to resist the apoptosis induction effects of FasL on CTL cells' surface and escape the cytotoxic attacks of CTL cells.[18] Some studies indicated that tumor cells' surface of melanoma did not express Fas; although there were Fas receptors on the surface of pancreas cancer cells, they could not conduct their functions.[19] On cell surface of human esophageal cancer and some kinds of leukemia, Fas expression was down-regulated.[20] In patients with liver cancer, Fas expression was up-regulated in normal liver cells, but down-regulated in liver cancer cells.[21] In these patients, the soluble Fas and FasL levels in serum were improved, indicating that liver cancer cells could escape immune surveillance in favor of their metastasis behaviors through eliminating their Fas expression, and letting liver cancer cells and invading monocytes produce soluble Fas.[21]
Up to now, there are few reports about the relation of the Fas/FasL system, clinical stages, and prognosis of NB. In this study, when the clinical stages of NB were deteriorated from I-II to IV-S and III-IV stage or their differentiation was reduced, Fas expression on tumor cell surfaces was gradually decreased. The postoperative survival time of Fas negative patients was significantly shorter than that of Fas positive patients, which was consistent with the result reported by Gross et al.[22] This indicates that some mechanisms may lead to the abnormal expression and function of the Fas system on tumor cell surfaces during the development of NB, in which case the Fas antigen could not transduce apoptosis signals. Thus apoptosis of tumor cells could not take place, resulting in the imbalance between apoptosis and proliferation. Besides, FasL expression in tissues of III-IV stage NB, the one with worst clinical prognosis, was significantly higher than that of I-II and IV-S stage NB. This indicates that NB tumor cells may also induce apoptosis of T-lymphocytes and escape immune surveillance by expressing FasL. To further investigate the role of the Fas/FasL system in the immune escape of NB, it is important to observe the effects of tumor cell-expressed FasL on apoptosis of Fas-sensitive T cells via cell culture and animal experiments. This study has proposed a new strategy for the treatment of NB. We could try to improve Fas expression on tumor cell surfaces, and down-regulate their FasL levels to treat NB. In summary, Fas/FasL abnormality may be one of the paths for NB to escape immune surveillance. Their expression detection was valuable not only in evaluating prognosis of NB patients, but also in exploring the etiological mechanisms and treatment methods of NB.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No.30200284) and Science Foundation of Huazhong University of Science and Technology.
Ethical approval: This study was approved by the Data Inspectorate of China and by the Ethical Committee of Tongji Medical College of China.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Tong QS proposed the study and wrote the first draft. Zheng LD analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. Tong QS is the guarantor.
References
1 Hug H. Fas-mediated apoptosis in tumor formation and defense. Boi Chem 1997;378:1405-1412.
2 Evans AE, D'Angio GJ, Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer 1971;27:374-378.
3 Shimada H, Chatten J, Newton WA Jr, Sachs N, Hamoudi AB, Chiba T, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst 1984;73:405-416.
4 Thapa L, He CM, Chen HP. Study on the expression of angiotensin II (ANG II) receptor subtype 1 (AT1R) in the placenta of pregnancy-induced hypertension. Placenta 2004;25:637-641.
5 Maris JM, Guo C, Blake D, White PS, Hogarty MD, Thompson PM, et al. Comprehensive analysis of chromosome 1p deletions in neuroblastoma. Med Pediatr Oncol 2001;36:32-36.
6 Joshi VV, Tsongalis GJ. Correlation between morphologic and nonmorphologic prognostic markers of neuroblastoma. Ann N Y Acad Sci 1997;824:71-83.
7 Schramm A, Schulte JH, Astrahantseff K, Apostolov O, Limpt V, Sieverts H, et al. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett 2005;228:143-153.
8 Terpe HJ, Christiansen H, Gonzalez M, Berthold F, Lampert F. Differentiation and prognosis of neuroblastoma in correlation to the expression of CD44s. Eur J Cancer 1995;31A:549-552.
9 Valteau-Couanet D, Leboulaire C, Maincent K, Tournier M, Hartmann O, Benard J, et al. Dendritic cells for NK/LAK activation: rationale for multicellular immunotherapy in neuroblastoma patients. Blood 2002;100:2554-2561.
10 Abrahams VM, Kamsteeg M, Mor G. The Fas/Fas ligand system and cancer: immune privilege and apoptosis. Mol Biotechnol 2003;25:19-30.
11 Houston A, O'Connell J. The Fas signalling pathway and its role in the pathogenesis of cancer. Curr Opin Pharmacol 2004;4:321-326.
12 Ferguson TA, Griffith TS. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev 2006;213:228-238.
13 Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Boil 2002;12:309-315.
14 Bohana-Kashtan O, Civin CI. Fas ligand as a tool for immunosuppression and generation of immune tolerance. Stem Cells 2004;22:908-924.
15 Kawasaki M, Kuwano K, Nakanishi Y, Hagimoto N, Takayama K, Pei XH, et al. Analysis of Fas and Fas ligand expression and function in lung cancer cell lines. Eur J Cancer 2000;36:656-663.
16 Yoshimura C, Nomura S, Kanazawa S, Kuwana M, Muramatsu M, Yamaguchi K, et al. Analysis of cytotoxic T lymphocytes and Fas/FasL in Japanese patients with non-small cell lung cancer associated with HLA-A2. J Cancer Res Clin Oncol 2002;128:581-588.
17 Gratas C, Tohma Y, Barnas C, Taniere P, Hainaut P, Ohgaki H. Up-regulation of Fas (APO-1/CD95) ligand and down-regulation of Fas expression in human esophageal cancer. Cancer Res 1998;58:2057-2062.
18 Rivoltini L, Radrizzani M, Accornero P, Squarcina P, Chiodoni C, Mazzocchi A, et al. Human melanoma-reactive CD4+ and CD8+ CTL clones resist Fas ligand-induced apoptosis and use Fas/Fas ligand-independent mechanisms for tumor killing. J Immunol 1998;161:1220-1230.
19 Bernstorff WV, Glickman JN, Odze RD, Farraye FA, Joo HG, Goedegebuure PS, et al. Fas (CD95/APO-1) and Fas ligand expression in normal pancreas and pancreatic tumors. Implications for immune privilege and immune escape. Cancer 2002;94:2552-2560.
20 Kase S, Osaki M, Adachi H, Kaibara N, Ito H. Expression of Fas and Fas ligand in esophageal tissue mucosa and carcinomas. Int J Oncol 2002;20:291-297.
21 Takehara T, Hayashi N. Fas and fas ligand in human hepatocellular carcinoma. J Gastroenterol 2001;36:727-728.
22 Gross N, Balmas K, Beretta Brognara C, Tschopp J. Expression of Fas (APO-1/CD95) and Fas ligand (FasL) in human neuroblastoma. Med Pediatr Oncol 2001;36:111-114.
Received January 8, 2007 Accepted after revision May 15, 2007
|

