| |
Clinical use of bisphosphonates in children
Laura Somalo, Fernando Santos
Oviedo, Spain
Author Affiliations: Department of Pediatrics, Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain (Somalo L, Santos F); Department of Medicine, Universidad de Oviedo, Oviedo, Asturias, Spain (Santos F); Instituto Universitario de Oncolog¨ªa del Principado de Asturias, Oviedo, Asturias, Spain (Santos F)
Corresponding Author: Fernando Santos, MD, PhD, Pediatria. Facultad de Medicina. c/ Julian Claveria 6, 33006 Oviedo, Asturias, Spain (Tel: +34985102728; Fax: +34985103585; Email: fsantos@uniovi.es)
Background: Bisphosphonates are being increasingly and successfully used to prevent bone fractures and treat bone pain in children with severe osteoporosis from different origins. The purpose of this article is to update the clinical use of bisphosphonates in pediatric patients and to present the authors' experience with the intravenous administration of pamidronate in osteopenic children.
Data sources: PubMed database was used to collect publications reporting the utilization of bisphosphonates in children. The medical records of five pediatric patients treated in our unit with bisphosphonates were also reviewed.
Results: A growing experience is accumulating with the cyclical intravenous administration of pamidronate in children with osteogenesis imperfecta. Bisphosphonates may also be useful in the prevention and treatment of vascular calcification in patients with chronic renal failure, although no data on children in this clinical setting are available.
Conclusions: Intravenous bisphosphonates are well tolerated, even in infants and small children, and represent a promising therapeutic tool to prevent the development of bone fractures and to improve the well-being of osteoporotic children. A number of questions about the precise clinical indications for bisphosphonates' administration, the duration of the treatment, the best way to monitor its effectiveness and to early detect toxic effects remain to be answered.
Key words: bisphosphonates; pamidronate; osteoporosis; osteogenesis imperfecta; calcification; chronic renal failure; hypercalcemia; bone diseases; kidney diseases
World J Pediatr 2007;3(4):245-253
Structure and mechanism of action
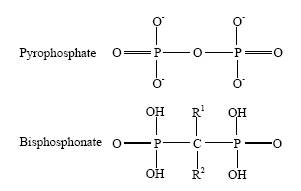
Bisphosphonates are analogues of pyrophosphate that contains two phosphonic acids directly attached to a central carbon atom, from which two side chains, denominated R1 and R2,[1] also extend (Fig. 1). Bisphosphonates bind avidly to the hydroxyapatite of bone surfaces and are subsequently accumulated in the osteoclasts by endocytosis. The R1 chain determines the affinity of this binding, whereas the R2 chain determines the potency of inhibiting bone resorption, which varies between different bisphosphonates up to 5000-fold, and the intrinsic mechanism of action on osteoclasts (Table 1). Bisphosphonates lead to osteoclasts' apoptosis by interfering with prenylation (transfer of fatty acid chains) of small guanosintriphosphate (GTP) binding proteins. Failure of prenylation causes inability of these proteins to translocate into cell membranes and results in apoptosis.[2] Older non-containing nitrogen bisphosphonates cause osteoclasts' apoptosis by forming toxic adenosintriphosphate (ATP) analogues. In addition to the bone resorption inhibition mediated by their effects on osteoclasts, bisphosphonates given in large doses inhibit normal and ectopic mineralization as a result of a direct chemical mechanism since they bind to solid phase calcium-phosphate and limit crystal growth.[1]
Fig. 1. Chemical structure of bisphosphonates.
Pharmacokinetics
Bisphosphonate compounds are available as intravenous or oral preparations (Table 1).[3] Digestive tolerability of oral bisphosphonates is poor and the experience with its use in children is really limited. To facilitate the absorption, which is less than 1% of the administered dose, bisphosphonates must be given in fasting conditions with enough amount of water. Absorption is negligible in the presence of divalent ions which chelate the bisphosphonates. Bisphosphonates circulate in the blood freely or weakly bind to plasma proteins. Unbound bisphosphonates are actively taken up by bone within 12-24 hours. Bisphosphonates bind preferentially to bones which have high turnover rates, and their distribution in bone is not homogeneous. After bone uptake, the bisphosphonates are liberated again only when the bone in which they are deposited is reabsorbed. Thus, the half-life of bisphosphonates in bone is very long, ranging among different species from 1 to 10 years, depending largely on the rate of bone turnover.[3] However, current data suggest that bisphosphonates are active only while binding to the bone surface, and become biologically inactive once the drug gets buried in bone tissue.[1] Bisphosphonates are eliminated unchanged in the urine and the doses should be adjusted in the case of renal insufficiency. In general, it is recommended to avoid the use of the majority of bisphosphonates when the glomerular filtration rate is below 30 ml/min/1.73 m2. The kinetics of bisphosphonates in children and patients with renal diseases is largely unknown.[4]
Table 1. Relative potency to inhibit bone reabsorption and side chain features of various bisphosphonates used in humans
|
Bisphosphonates |
Antiresorptive potency |
R1 |
R2 |
|
Etidronate |
1 |
OH |
CH3 |
|
Clodronate |
10 |
Cl |
Cl |
|
Tiludronate |
10 |
H |
S-CH4Cl |
|
Pamidronate |
100 |
OH |
Contains nitrogen |
|
Neridronate |
100 |
OH |
Contains nitrogen |
|
Olpadronate |
200¨C500 |
OH |
Contains nitrogen |
|
Ibandronate |
500¨C1000 |
OH |
Contains nitrogen |
|
Alendronate |
1000¨C2000 |
OH |
Contains nitrogen |
|
Risedronate |
2000 |
OH |
Contains nitrogen |
|
Zoledronate |
10000 |
OH |
Contains nitrogen |
Clinical use in children
In adults, the potent ability of bisphosphonates to inhibit bone reabsorption has been extensively used over the last three decades to treat osteoporosis, Paget's disease, hypercalcemia of malignancy, tumor-induced bone disease, and hyperparathyroidism. More preliminary experimental and clinical data indicate that intravenous bisphosphonates may be useful in the treatment and prevention of atherosclerosis because they reduce arterial calcification and improve the serum lipoprotein profile. This beneficial effect on the concentrations of serum lipoprotein is related to the ability of aminobisphosphonates to inhibit the mevalonate pathway and so to interfere with cholesterol synthesis. A shift in circulating cholesterol from low-density lipoprotein cholesterol to high-density lipoprotein cholesterol and marginal decrease in total serum cholesterol and triglycerides has been found in postmenopausal women with osteoporosis[5] and patients with Paget's disease[6] treated with aminobisphosphonates.
The experience with bisphosphonates in children is limited although there are a growing number of publications showing their usefulness in several bone and metabolic diseases. The largest population of pediatric patients treated with bisphosphonates corresponds to children with osteogenesis imperfecta. In 1998, Glorieux et al[7] reported that intravenous administration of pamidronate at 4 to 6-month intervals improved bone mineral density, mobility, incidence of bone fractures and reduced chronic bone pain and biochemical markers of bone resorption in 30 children with severe osteogenesis imperfecta. This study indicated the beneficial effects of bisphosphonates in this disease as demonstrated by former clinical observations,[8-10] and prompted the increasing use of this therapy in children with moderate and severe osteogenesis imperfecta.[11-46] In these studies, the vast majority coming from the same group,[7,13,16-20,29,36,37,41,44,45] cyclical intravenous administration of pamidronate was given most frequently, although a few studies have reported the effects of other bisphosphonates such as oral alendronate,[26-28,33] oral olpadronate,[24] or intravenous neridronate.[32,38]
Nowadays, the frequently recommended dosage of pamidronate in children with osteogenesis imperfecta is 1 mg/kg per day, infused intravenously with 0.9% saline solution in a 4-hour period for three consecutive days every 4 months.[45] In infants, lower dose of pamidronate is recommended at shorter intervals, such as 0.5 mg/kg per day in 3-day cycles every 6-8 weeks[20,29] because of the shorter duration of the clinical effects in this age group. To test the tolerance to the medication, the dose used in the first cycle must be lower than those of the above in all patients.[45]
The experience with the use of bisphosphonates in pediatric patients with diseases other than osteogenesis imperfecta is mostly based on small series or single clinical cases. Thus bisphosphonates have been used in the treatment of hypercalcemia induced by such conditions as cancer,[47-56] bone marrow transplantation for osteopetrosis,[57] prolonged immobilization,[58-60] vitamin A toxicity,[61] total parenteral nutrition,[62-64] Williams syndrome,[65] vitamin D intoxication,[66-70] or hyperparathyroidism.[59]
Interestingly, bisphosphonates are administered to prevent the development of fractures in children with osteoporosis secondary to prolonged immobilization caused by neurological or muscular diseases. Allington et al[71] treated 18 osteoporotic children with non-ambulatory cerebral palsy and other neuromuscular disorders with intravenous administration of pamidronate in cycles of 3 mg/kg every four months. They found improvement in bone densitometry, particularly in the lumbar spine, as well as beneficial clinical effects such as decrease of pain on manipulation and absence of new fractures after one year of treatment. Similar results were observed after the cyclical intravenous administration of pamidronate in children with cerebral palsy[34] and of pamidronate or olpadronate in children with different symptomatic osteoporosis and multiple fractures.[72] A positive response to oral alendronate of total body and spine bone mineral density was found in 16 deflazacort-treated boys with Duchenne muscular dystrophy.[73] Moreover, the administration of alendronate markedly decreased the incidence of fractures in 10 non-ambulatory children with disuse osteopenia secondary to either static brain injury or spina bifida.[74] Once-weekly oral alendronate has been shown to increase the volumetric bone density of the lumbar spine and reduce urine N-telopeptide excretion as a biochemical marker of bone resorption in children treated persistently with glucocorticoids.[75] In a group of 17 osteopenic or osteoporotic children, administration of pamidronate or zoledronic acid (1 mg of pamidronate = 0.025 mg of zoledronic acid) for 6-43 months showed a beneficial effect on bone mineral density and, more importantly, the effect maintained for 18-44 months after withholding the treatment.[76]
Bisphosphonates have been used in the treatment of calcinosis and heterotopic ossification in children with dermatomyositis,[77-80] myositis ossificans,[81-84] and fibrodysplasia ossificans progressiva.[85-87] The majority of these studies are based on the administration of etidronate disodium in a single center with few clinical cases, and the results are variable. Recent reports have shown rapid and marked improvement of calcinosis in two children with dermatomyositis treated with alendronate.[79,80]
The intravenous administration of pamidronate in children in our institute is analyzed (Table 2). Five osteoporotic patients, 3 male, aged 2 to 14 years at the beginning of treatment, received intravenous pamidronate at 2-3 mg/kg per cycle for 3-day cycles. Four of them had presented fractures caused by minor or absent trauma. Immediately before each cycle, serum concentrations of calcium, phosphate, alkaline phosphatase, blood urea nitrogen, creatinine, osteocalcin, PTH and vitamin D metabolites were measured as well as blood acid-base equilibrium. Elemental uroanalysis as well as measurement of the concentrations of cross-linked N telopeptide type I collagen, hydroxiproline, calcium, and creatinine were performed in the second urine sample collected in the morning after fasting the first day of each cycle. Bone mineral density was measured before the treatment and every one to two years afterwards. All children had a positive response to the treatment, with rapid and marked clinical improvement in their mobility and strength. Moreover, one patient who was confined to bed before treatment became able to walk. After receiving two or three cycles of pamidronate, no new fractures occurred during follow-up of 6-7 months in two children who subsequently died from primary disease related complications. Patients' tolerance to treatment was excellent, fever was noted in 3 of the 5 patients after administration of the first dose of pamidronate for the first cycle. Consistent and significant changes in the above mentioned biochemical variables were not found except the level of urine N telopeptide type I collagen. In patients followed up for more than one year, the levels of N telopeptide type I collagen decreased to 40% of the initial levels after the third cycle of pamidronate and were still between 30% to 40% of the pre-treatment levels at the last follow-up. Hypocalcemia and proteinuria were not detected in any patient. Bone mineral density was improved in the 3 patients, in whom it could be repeatedly measured.
Table 2. Clinical features of five osteoporotic children treated with intravenous cycles of pamidronate
|
Patient |
1 |
2 |
3 |
4 |
5 |
|
Sex |
Male |
Male |
Female |
Male |
Female |
|
Age (y) |
7 |
14 |
12 |
2 |
9 |
|
Primary diagnosis |
Osteogenesis
imperfecta type III |
Cerebral palsy |
Idiopathic
osteoporosis |
Spinal muscular
atrophy |
Severe myopathy |
|
Associated diagnosis |
Urolithiasis |
Epilepsy, vesicoureteral
reflux |
Asthma |
Chronic respiratory
insufficiency |
Chronic respiratory insufficiency,
malnutrition |
|
Follow up |
7 y |
6 mon |
5 y |
2.5 y |
7 mon |
|
Cycles of pamidronate |
27 |
2 |
7 |
11 |
3 |
|
Fractures before treatment |
14 |
2 |
6 |
0 |
2 |
|
Fractures during follow-up |
0 |
0 |
0 |
0 |
0 |
|
Initial weight (kg) (SDS) |
11.3 (-2.9) |
28 (-2.3) |
55 (+1.5) |
10.7 (-1.5) |
14.0 (-2.7) |
|
Final weight (kg) (SDS) |
25.5 (-2.5) |
29 (-2.2) |
70 (+2.5) |
20 (+1.5) |
16 (-2.6) |
|
Initial height (cm) (SDS) |
84 (-) |
134 (-3.6) |
157 (+0.8) |
86.5 (-0.06) |
120 (-2.4) |
|
Final height (cm) (SDS) |
141 (-) |
Not available |
162 (+0.9) |
100 (-0.6) |
Not available |
|
Initial BMD (g/cm2) (SDS) |
180 (-) |
0.455 (-5.9) |
0.666 (-3.4) |
0.326 (-3.0) |
0.459 (-2.4) |
|
Final BMD (g/cm2) (SDS) |
400 (-) |
Not available |
0.969 (-1.2) |
0.541 (+0.45) |
Not available |
|
Side effects |
Fever |
Fever |
None |
Fever |
None |
|
Clinical outcome |
Improvement |
Improvement |
Improvement |
Not available |
Improvement | BMD: bone mineral density; SDS: standard deviation score. Lumbar spine BMD was measured by DEXA, except for patient 1 in whom it was measured on forearm because of calcium nephrolithiasis. For patient 1, SDS of height and BMD were not provided because of the lack of adequate normal reference values.
Bisphosphonates in kidney diseases
The potent inhibitory effect of bisphosphonates on bone reabsorption has been implicated in renal transplanted adults to prevent or treat bone loss induced by persistent administration of glucocorticoids.[88-102] Two randomized controlled trials on bisphosphonates in kidney transplant recipients[99,100] revealed that the treatment with bisphosphonates has a beneficial effect on bone mineral density although the trials were inadequately powered to show a reduction in the risk for fracture. The experience with the use of bisphosphonates in transplanted children is very limited. In 15 osteopenic or osteoporotic patients transplanted at the age of 17 years or less, the T-score of bone mineral density at the lumbar spine changed from -2.3 to -1.9 after 12 months of oral treatment with alendronate at a dose of 5 mg/day, whereas the density fell from -2.4 to -2.8 in untreated transplanted controls and improved from -2.3 to -0.5 and from -2.3 to 1.0 in patients receiving either alphacalcidiol (0.25 mcg/day) or nasal spray calcitonin (200 IU/day).[103] In pediatric patients with nephropathies receiving high doses of corticosteroids, administration of 125 mg of pamidronate for 3 months prevented the loss of lumbar spine bone mineral density induced by the steroid treatment.[104]
Because of their inhibitory activity of bone resorption and ability to interfere with mineralization and calcium phosphate crystallization,[105] bisphosphonates might be useful in the management of selected hypercalciuric patients with urolithiasis.[106] A 2-year therapy with etidronate increased bone mineral density in lumbar spine of young male patients with idiopathic hypercalciuria and osteopenia.[107] Alendronate has been shown to decrease urine calcium and super-saturation of urine with respect to calcium oxalate and calcium hydrogen phosphate in genetic hypercalciuric stone-forming rats[108] and adult humans subjected to bed rest immobilization.[109]
Bisphosphonates are given to adult patients with severe hyperparathyroidism secondary to end-stage renal failure in an attempt to reduce hypercalcemia caused by increased bone reabsorption and therapy with vitamin D analogues and/or calcium containing phosphate binders.[110-116] The results of the studies, always based on a small number of patients, do not support the use of bisphosphonates in the treatment of secondary hyperparathyroidism because of the lack of uniformity in the effects on serum calcium concentration, the presence of mineralization defects, and changes in the bone histological structure, such as increase of both fibrous tissue and the number of osteoclasts,[111,114] and even worsening of serum parathyroid hormone.[110,116] However, in the experimental model of chronic renal failure induced by subtotal nephrectomy, ibandronate[117] and olpadronate[118] have been shown to increase bone volume and prevent the osseous lesions of hyperparathyroidism.
More interestingly, bisphosphonates are potentially used to inhibit ectopic mineralization for the prevention and treatment of vascular calcifications in patients with end-stage renal failure. Several experimental studies have been carried out on rats with acute uremia,[119] rats with normal renal function receiving a toxic dose of vitamin D,[120] subtotally nephrectomized rats treated with calcitriol for three weeks,[121] and rats made uremic by administration of a synthetic diet containing 0.75% of adenine for 4 weeks.[122] In those studies, all the animals treated with bisphosphonates demonstrated a lower calcium content in the aorta. In a diabetic woman with chronic renal failure, intravenous administration of pamidronate improved dramatically calciphylaxis, a condition characterized by medial calcification of the small arteries and ischemia of the subcutaneous tissue, often leading to necrosis of subcutaneous fat and skin.[123] Likewise, oral treatment with etidronate for 3 cycles given for 14 days every three months ameliorated the progression of coronary artery calcification, as assessed by multidetector spiral computed tomography in 26 of 36 adult patients receiving frequent hemodialysis.[124]
Side effects and toxicity
Oral administration of bisphosphonates produces some degree of gastrointestinal intolerance, particularly the aminobisphosphonates. To minimize esophageal irritation, they must be given in fasting conditions and with large amounts of water which limit their use in young children and patients with swallowing difficulties or obliged to remain in decubitus. Administration of risendronate or alendronate once a week facilitates the oral tolerance. Etidronate does not contain nitrogen and is usually well tolerated, causing mild diarrhea.
In children, pamidronate is given cyclically by intravenous infusion, giving few serious adverse events. After the first dose for the first cycle, one third of patients may undergo an immediate reaction characterized by high fever, malaise, musculoskeletal aches and lymphocytopenia. The reaction usually does not recur in further cycles, and can be prevented by pre-treatment with acetaminophen and ibuprofen,[125] and may also be occasionally observed with oral administration of bisphosphonates in a milder intensity.[126]
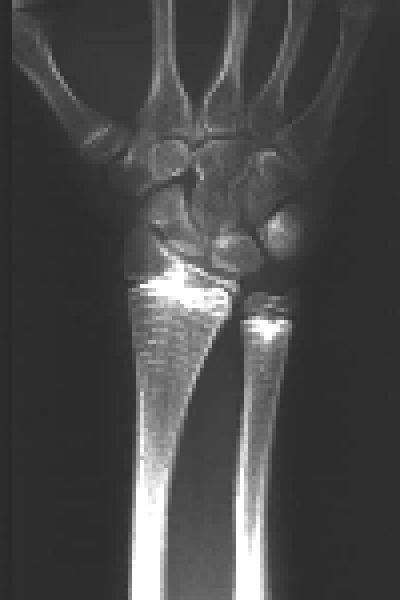
Given the potent effect of pamidronate on osseous metabolism, several studies have analyzed whether the repeated administration of this drug might adversely impair growth or bone structure. Each cycle of pamidronate leaves a radiologically visible metaphyseal band (Fig. 2). Sclerotic lines are formed by trabeculae rich in calcified cartilage, and the percentage of calcified cartilage decreases as the band goes away from the growth plate.[127] The trabeculae undergo remodeling and disappear within 2 to 8 years, and likely represent a non-specific effect resulting from temporary interruption of growth plate cartilage resorption at the time of pamidronate infusion.[127,128] A widening of the distal femoral metaphyses was seen on radiographs of children with osteogenesis imperfecta who had received pamidronate therapy for 2-4 years.[128] This change in the methaphyses is attributable to the interference of pamidronate with the physiological process of periosteal resorption. In spite of these radiological findings, longitudinal growth rate is not adversely affected by pamidronate treatment in osteoporotic children.[72] As to the bone structure, mineralization defects have not been observed in patients with osteoporosis[72] or osteogenesis imperfecta[13] treated with pamidronate. Histomorphometric analysis of iliac bone of infants, children and adolescents with osteogenesis imperfecta indicates that pamidronate increases cortical width and the volume of cancellous bone and suppresses bone turnover markedly.[13,29] Excessive amounts of pamidronate may lead to oversuppression of bone resorption and osteopetrosis, whereby monitoring of biochemical markers of skeletal turnover is mandatory in children treated with bisphosphonates.[129]
Fig. 2. Methaphyseal bands, each corresponding to a cycle of pamidronate, are visible in the wrist X-ray of a patient with osteogenesis imperfecta.
Hypocalcemia may result from pamidronate administration. It is usually mild and asymptomatic,[45,65] and the occurrence of more severe hypocalcemia must suggest unrecognized hypoparathyroidism, impaired renal function, or vitamin D deficiency as underlying conditions.[130]
Adverse effects of pamidronate found in adult patients such as osteonecrosis of the jaw in patients with cancer,[131] collapsing focal segmental glomerulosclerosis leading to nephrotic syndrome,[132] and acute tubular necrosis[133,134] have not been reported in children so far.
In summary, a growing number of publications support the use of bisphosphonates in children with different bone disorders to improve bone mineral density, reduce the risk of fracture and ameliorate bone related clinical symptoms. Osteogenesis imperfecta is the disease for which bisphosphonates are extensively used and intravenous pamidronate is often employed cyclically. The metabolism of calcium, phosphate, vitamin D and parathyroid hormone as well as biochemical markers of bone turnover, particularly bone resorption, should be closely monitored before and during the administration of bisphosphonates. Some questions concerning the clinical use of bisphosphonates remain to be answered. Should osteoporotic children receive bisphosphonates in a prophylactic way to prevent the development of fractures? When should the treatment be started and for how long? Which protocol and drug should be recommended in the different clinical settings? Which are the long-term effects of bisphosphonates' administration? Which is the best way to monitor the effectiveness of treatment?
Funding: This study was partly supported by Nutrici¨®n y Crecimiento Foundation, Spain.
Ethical approval: Not needed.
Competing interest: None declared.
Contributors: SF wrote the main body of the article. SL reviewed the medical records of the patients from our institution and analyzed the collected data.
References
1 Vasikaran SD. Bisphosphonates: an overview with special reference to alendronate. Ann Clin Biochem 2001;38:608-623.
2 Ortiz-Gomez A, Jimenez C, Estevez AM, Carrero-Lerida J, Ruiz-Perez LM, Gonzalez-Pacanowska D. Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overexpression confers resistance to risedronate. Eukaryot Cell 2006;5:1057-1064.
3 Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone 1996;18:75-85.
4 Rodd C. Bisphosphonates in dialysis and transplantation patients: efficacy and safety issues. Perit Dial Int 2001;21 Suppl 3:S256-260.
5 Adami S, Braga V, Guidi G, Gatti D, Gerardi D, Fracassi E. Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. J Bone Miner Res 2000;15:599-604.
6 Montagnani A, Gonnelli S, Cepollaro C, Campagna MS, Franci MB, Pacini S, et al. Changes in serum HDL and LDL cholesterol in patients with Paget's bone disease treated with pamidronate. Bone 2003;32:15-19.
7 Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 1998;339:947-952.
8 Devogelaer JP, Malghem J, Maldague B, Nagant de Deuxchaisnes C. Radiological manifestations of bisphosphonate treatment with APD in a child suffering from osteogenesis imperfecta. Skeletal Radiol 1987;16:360-363.
9 Landsmeer-Beker EA, Massa GG, Maaswinkel-Mooy PD, van de Kamp JJ, Papapoulos SE. Treatment of osteogenesis imperfecta with the bisphosphonate olpadronate (dimethylaminohydroxypropylidene bisphosphonate). Eur J Pediatr 1997;156:792-794.
10 Astrom E, Soderhall S. Beneficial effect of bisphosphonate during five years of treatment of severe osteogenesis imperfecta. Acta Paediatr 1998;87:64-68.
11 Gonzalez E, Pavia C, Ros J, Villaronga M, Valls C, Escola J. Efficacy of low dose schedule pamidronate infusion in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab 2001;14:529-533.
12 Lee YS, Low SL, Lim LA, Loke KY. Cyclic pamidronate infusion improves bone mineralisation and reduces fracture incidence in osteogenesis imperfecta. Eur J Pediatr 2001;160:641-644.
13 Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest 2002;110:1293-1299.
14 Giraud F, Meunier PJ. Effect of cyclical intravenous pamidronate therapy in children with osteogenesis imperfecta. Open-label study in seven patients. Joint Bone Spine 2002;69:486-490.
15 Astrom E, Soderhall S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child 2002;86:356-364.
16 Montpetit K, Plotkin H, Rauch F, Bilodeau N, Cloutier S, Rabzel M, et al. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics 2003;111:e601-603.
17 Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics 2003;111:1030-1036.
18 Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res 2003;18:610-614.
19 Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH. Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab 2003;88:986-992.
20 Plotkin H, Rauch F, Bishop NJ, Montpetit K, Ruck-Gibis J, Travers R, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab 2000;85:1846-1850.
21 Falk MJ, Heeger S, Lynch KA, DeCaro KR, Bohach D, Gibson KS, et al. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics 2003;111:573-578.
22 DiMeglio LA, Ford L, McClintock C, Peacock M. Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone 2004;35:1038-1045.
23 Zacharin M, Kanumakala S. Pamidronate treatment of less severe forms of osteogenesis imperfecta in children. J Pediatr Endocrinol Metab 2004;17:1511-1517.
24 Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet 2004;363:1427-1431.
25 Arikoski P, Silverwood B, Tillmann V, Bishop NJ. Intravenous pamidronate treatment in children with moderate to severe osteogenesis imperfecta: assessment of indices of dual-energy X-ray absorptiometry and bone metabolic markers during the first year of therapy. Bone 2004;34:539-546.
26 Seikaly MG, Kopanati S, Salhab N, Waber P, Patterson D, Browne R, et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. J Pediatr Orthop 2005;25:786-791.
27 Vyskocil V, Pikner R, Kutilek S. Effect of alendronate therapy in children with osteogenesis imperfecta. Joint Bone Spine 2005;72:416-423.
28 Cho TJ, Choi IH, Chung CY, Yoo WJ, Park MS, Park YK. Efficacy of oral alendronate in children with osteogenesis imperfecta. J Pediatr Orthop 2005;25:607-612.
29 Munns CF, Rauch F, Travers R, Glorieux FH. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical and histomorphometric outcome. J Bone Miner Res 2005;20:1235-1243.
30 Forin V, Arabi A, Guigonis V, Filipe G, Bensman A, Roux C. Benefits of pamidronate in children with osteogenesis imperfecta: an open prospective study. Joint Bone Spine 2005;72:313-318.
31 Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res 2005;20:977-986.
32 Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tato L, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res 2005;20:758-763.
33 Pizones J, Plotkin H, Parra-Garcia JI, Alvarez P, Gutierrez P, Bueno A, et al. Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. J Pediatr Orthop 2005;25:332-335.
34 Grissom LE, Kecskemethy HH, Bachrach SJ, McKay C, Harcke HT. Bone densitometry in pediatric patients treated with pamidronate. Pediatr Radiol 2005;35:511-517.
35 DiMeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. J Pediatr Endocrinol Metab 2005;18:43-53.
36 Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone 2006;39:901-906.
37 Weber M, Roschger P, Fratzl-Zelman N, Schoberl T, Rauch F, Glorieux FH, et al. Pamidronate does not adversely affect bone intrinsic material properties in children with osteogenesis imperfecta. Bone 2006;39:616-622.
38 Antoniazzi F, Zamboni G, Lauriola S, Donadi L, Adami S, Tato L. Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. J Pediatr 2006;149:174-179.
39 El-Sobky MA, Hanna AA, Basha NE, Tarraf YN, Said MH. Surgery versus surgery plus pamidronate in the management of osteogenesis imperfecta patients: a comparative study. J Pediatr Orthop 2006;15:222-228.
40 Madenci E, Yilmaz K, Yilmaz M, Coskun Y. Alendronate treatment in osteogenesis imperfecta. J Clin Rheumatol 2006;12:53-56.
41 Land C, Rauch F, Montpetit K, Ruck-Gibis J, Glorieux FH. Effect of intravenous pamidronate therapy on functional abilities and level of ambulation in children with osteogenesis imperfecta. J Pediatr 2006;148:456-460.
42 Goksen D, Coker M, Darcan S, Kose T, Kara S. Low-dose intravenous pamidronate treatment in osteogenesis imperfecta. Turk J Pediatr 2006;48:124-129.
43 Vallo A, Rodriguez-Leyva F, Rodriguez Soriano J. Osteogenesis imperfecta: anthropometric, skeletal and mineral metabolic effects of long-term intravenous pamidronate therapy. Acta Paediatr 2006;95:332-339.
44 Rauch F, Travers R, Glorieux FH. Pamidronate in children with osteogenesis imperfecta: histomorphometric effects of long-term therapy. J Clin Endocrinol Metab 2006;91:511-516.
45 Zeitlin L, Rauch F, Travers R, Munns C, Glorieux FH. The effect of cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta type V. Bone 2006;38:13-20.
46 DiMeglio LA, Peacock M. Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. J Bone Miner Res 2006;21:132-140.
47 Boudailliez BR, Pautard BJ, Sebert JL, Kremp O, Piussan CX. Leukaemia-associated hypercalcaemia in a 10-year-old boy: effectiveness of aminohydroxypropylidene biphosphonate. Pediatr Nephrol 1990;4:510-511.
48 Kutluk MT, Hazar V, Akyuz C, Varan A, Buyukpamukcu M. Childhood cancer and hypercalcemia: report of a case treated with pamidronate. J Pediatr 1997;130:828-831.
49 Young G, Shende A. Use of pamidronate in the management of acute cancer-related hypercalcemia in children. Med Pediatr Oncol 1998;30:117-121.
50 De Schepper J, de Pont S, Smitz J, De Coster D, Schots R, Otten J. Metabolic disturbances after a single dose of 30 mg pamidronate for leukaemia-associated hypercalcaemia in a 11-year-old boy. Eur J Pediatr 1999;158:765-766.
51 Schmid I, Stachel D, Schon C, Bauer M, Haas RJ. Pamidronate and calcitonin as therapy of acute cancer-related hypercalcemia in children. Klin Pediatr 2001;213:30-34.
52 Sakamoto O, Yoshinari M, Rikiishi T, Fujiwara I, Imaizumi M, Tsuchiya S, et al. Hypercalcemia due to all-trans retinoic acid therapy for acute promyelocytic leukemia: a case report of effective treatment with bisphosphonate. Pediatr Int 2001;43:688-690.
53 Mathur M, Sykes JA, Saxena VR, Rao SP, Goldman GM. Treatment of acute lymphoblastic leukemia-induced extreme hypercalcemia with pamidronate and calcitonin. Pediatr Crit Care Med 2003;4:252-255.
54 Buonuomo PS, Ruggiero A, Piastra M, Riccardi R, Polidori G, Chiaretti A. A case of acute lymphoblastic leukemia presenting as severe hypercalcemia. Pediatr Hematol Oncol 2004;21:475-479.
55 Kerdudo C, Aerts I, Fattet S, Chevret L, Pacquement H, Doz F, et al. Hypercalcemia and childhood cancer: a 7-year experience. J Pediatr Hematol Oncol 2005;27:23-27.
56 Andiran N, Alikasifoglu A, Kupeli S, Yetgin S. Use of bisphosphonates for resistant hypercalcemia in children with acute lymphoblastic leukemia: report of two cases and review of the literature. Turk J Pediatr 2006;48:248-252.
57 Rawlinson PS, Green RH, Coggins AM, Boyle IT, Gibson BE. Malignant osteopetrosis: hypercalcaemia after bone marrow transplantation. Arch Dis Child 1991;66:638-639.
58 Profumo RJ, Reese JC, Foy TM, Garibaldi LR, Kane RE. Severe immobilization-induced hypercalcemia in a child after liver transplantation successfully treated with pamidronate. Transplantation 1994;57:301-303.
59 Sellers E, Sharma A, Rodd C. The use of pamidronate in three children with renal disease. Pediatr Nephrol 1998;12:778-781.
60 Go T. Low-dose oral etidronate therapy for immobilization hypercalcaemia associated with Guillain-Barre syndrome. Acta Paediatr 2001;90:1202-1204.
61 Doireau V, Macher MA, Brun P, Bernard O, Loirat C. Vitamin A poisoning revealed by hypercalcemia in a child with kidney failure. Arch Pediatr 1996;3:888-890.
62 Attard TM, Dhawan A, Kaufman SS, Collier DS, Langnas AN. Use of disodium pamidronate in children with hypercalcemia awaiting liver transplantation. Pediatr Transplant 1998;2:157-159.
63 Duke JL, Jones DP, Frizzell NK, Chesney RW, Hak EB. Pamidronate in a girl with chronic renal insufficiency dependent on parenteral nutrition. Pediatr Nephrol 2003;18:714-717.
64 Bryowsky JJ, Bugnitz MC, Hak EB. Pamidronate treatment for hypercalcemia in an infant receiving parenteral nutrition. Pharmacotherapy 2004;24:939-944.
65 Cagle AP, Waguespack SG, Buckingham BA, Shankar RR, Dimeglio LA. Severe infantile hypercalcemia associated with Williams syndrome successfully treated with intravenously administered pamidronate. Pediatrics 2004;114:1091-1095.
66 Bereket A, Erdogan T. Oral bisphosphonate therapy for vitamin D intoxication of the infant. Pediatrics 2003;111:899-901.
67 Gurkan F, Davutoglu M, Bosnak M, Ece A, Dikici B, Bilici M, et al. Pamidronate treatment in acute vitamin D intoxication. J Endocrinol Invest 2004;27:680-682.
68 Ezgu FS, Buyan N, Gunduz M, Tumer L, Okur I, Hasanoglu A. Vitamin D intoxication and hypercalcaemia in an infant treated with pamidronate infusions. Eur J Pediatr 2004;163:163-165.
69 Hatun S, Cizmecioglu F. Use of alendronate in the treatment of vitamin D intoxication in infants. Turk J Pediatr 2005;47:373-375.
70 Atabek ME, Pirgon O, Sert A. Oral alendronate therapy for severe vitamin D intoxication of the infant with nephrocalcinosis. J Pediatr Endocrinol Metab 2006;19:169-172.
71 Allington N, Vivegnis D, Gerard P. Cyclic administration of pamidronate to treat osteoporosis in children with cerebral palsy or a neuromuscular disorder: a clinical study. Acta Orthop Belg 2005;71:91-97.
72 Brumsen C, Hamdy NA, Papapoulos SE. Long-term effects of bisphosphonates on the growing skeleton. Studies of young patients with severe osteoporosis. Medicine (Baltimore) 1997;76:266-283.
73 Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil 2005;86:284-288.
74 Sholas MG, Tann B, Gaebler-Spira D. Oral bisphosphonates to treat disuse osteopenia in children with disabilities: a case series. J Pediatr Orthop 2005;25:326-331.
75 Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology (Oxford) 2005;44:813-818.
76 Waterhouse KM, Auron A, Srivastava T, Haney C, Alon US. Sustained beneficial effect of intravenous bisphosphonates after their discontinuation in children. Pediatr Nephrol 2007;22:282-287.
77 Goel KM, Shanks RA. Dermatomyositis in childhood. Review of eight cases. Arch Dis Child 1976;51:501-506.
78 Weinstein RS. Focal mineralization defect during disodium etidronate treatment of calcinosis. Calcif Tissue Int 1982;34:224-228.
79 Mukamel M, Horev G, Mimouni M. New insight into calcinosis of juvenile dermatomyositis: a study of composition and treatment. J Pediatr 2001;138:763-766.
80 Ambler GR, Chaitow J, Rogers M, McDonald DW, Ouvrier RA. Rapid improvement of calcinosis in juvenile dermatomyositis with alendronate therapy. J Rheumatol 2005;32:1837-1839.
81 Smith R, Russell RG, Woods CG. Myositis ossificans progressiva. Clinical features of eight patients and their response to treatment. J Bone Joint Surg Br 1976;58:48-57.
82 Sanchez E, Maturana I, Delgado A. Progressive myositis ossificans. Report of a case and results of treatment with disodium etidronate. An Esp Pediatr 1984;21:597-601.
83 Bar Oz B, Boneh A. Myositis ossificans progressiva: a 10-year follow-up on a patient treated with etidronate disodium. Acta Paediatr 1994;83:1332-1334.
84 Alpigiani MG, Puleo MG, Callegarini L, Di Bella E, Debbia C, Buzzanca C, et al. Dichloromethylenbiphosphonic acid in the therapy of myositis ossificans progressive (MOP). Minerva Pediatr 1996;48:159-163.
85 Rogers JG, Dorst JP, Geho WB. Use and complications of high-dose disodium etidronate therapy in fibrodysplasia ossificans progressiva. J Pediatr 1977;91:1011-1014.
86 Bruni L, Giammaria P, Tozzi MC, Camparcola D, Scopinaro F, Imperato C. Fibrodysplasia ossificans progressiva. An 11-year-old boy treated with a diphosphonate. Acta Paediatr Scand 1990;79:994-998.
87 Dua T, Kabra M, Kalra V. Familial fibrodysplasia ossificans progressiva: trial with etidronate disodium. Indian Pediatr 2001;38:1305-1309.
88 Fan SL, Almond MK, Ball E, Evans K, Cunningham J. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int 2000;57:684-690.
89 Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, et al. Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol 2001;12:1530-1537.
90 Arlen DJ, Lambert K, Ioannidis G, Adachi JD. Treatment of established bone loss after renal transplantation with etidronate. Transplantation 2001;71:669-673.
91 Koc M, Tuglular S, Arikan H, Ozener C, Akoglu E. Alendronate increases bone mineral density in long-term renal transplant recipients. Transplant Proc 2002;34:2111-2113.
92 Cruz DN, Brickel HM, Wysolmerski JJ, Gundberg CG, Simpson CA, Kliger AS, et al. Treatment of osteoporosis and osteopenia in long-term renal transplant patients with alendronate. Am J Transplant 2002;2:62-67.
93 Torregrosa JV, Moreno A, Gutierrez A, Vidal S, Oppenheimer F. Alendronate for treatment of renal transplant patients with osteoporosis. Transplant Proc 2003;35:1393-1395.
94 Fan SL, Kumar S, Cunningham J. Long-term effects on bone mineral density of pamidronate given at the time of renal transplantation. Kidney Int 2003;63:2275-2279.
95 Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Mitterbauer C, et al. Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int 2003;63:1130-1136.
96 Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 2003;14:2669-2676.
97 Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: a randomized prospective trial of calcitriol versus alendronate. Transplantation 2003;76:1498-1502.
98 Schwarz C, Mitterbauer C, Heinze G, Woloszczuk W, Haas M, Oberbauer R. Nonsustained effect of short-term bisphosphonate therapy on bone turnover three years after renal transplantation. Kidney Int 2004;65:304-309.
99 Palmer SC, Strippoli GF, McGregor DO. Interventions for preventing bone disease in kidney transplant recipients: a systematic review of randomized controlled trials. Am J Kidney Dis 2005;45:638-649.
100 Mitterbauer C, Schwarz C, Haas M, Oberbauer R. Effects of bisphosphonates on bone loss in the first year after renal transplantation¡ªa meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2006;21:2275-2281.
101 El-Agroudy AE, El-Husseini AA, El-Sayed M, Mohsen T, Ghoneim MA. A prospective randomized study for prevention of postrenal transplantation bone loss. Kidney Int 2005;67:2039-2045.
102 Nowacka-Cieciura E, Cieciura T, Baczkowska T, Kozinska-Przybyl O, Tronina O, Chudzinski W, et al. Bisphosphonates are effective prophylactic of early bone loss after renal transplantation. Transplant Proc 2006;38:165-167.
103 El-Husseini AA, El-Agroudy AE, El-Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatr Transplant 2004;8:357-361.
104 Kim SD, Cho BS. Pamidronate therapy for preventing steroid-induced osteoporosis in children with nephropathy. Nephron Clin Pract 2006;102:c81-87.
105 Oata M, Pak CY. The effect of diphosphonate on calcium phosphate crystallization in urine in vitro. Kidney Int 1973;4:401-406.
106 Vaidyanathan S, Watson ID, Jonsson O, Buczynski AZ, Grases F, Heilberg IP, et al. Recurrent vesical calculi, hypercalciuria, and biochemical evidence of increased bone resorption in an adult male with paraplegia due to spinal cord injury: is there a role for intermittent oral disodium etidronate therapy for prevention of calcium phosphate bladder stones? Spinal Cord 2005;43:269-277.
107 Heilberg IP, Martini LA, Teixeira SH, Szejnfeld VL, Carvalho AB, Lobao R, et al. Effect of etidronate treatment on bone mass of male nephrolithiasis patients with idiopathic hypercalciuria and osteopenia. Nephron 1998;79:430-437.
108 Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 1999;55:234-243.
109 Ruml LA, Dubois SK, Roberts ML, Pak CY. Prevention of hypercalciuria and stone-forming propensity during prolonged bedrest by alendronate. J Bone Miner Res 1995;10:655-662.
110 Hamdy NA, McCloskey EV, Brown CB, Kanis JA. Effects of clodronate in severe hyperparathyroid bone disease in chronic renal failure. Nephron 1990;56:6-12.
111 Hene RJ, Visser WJ, Duursma SA, Raymakers JA, de bos Kuil RJ, Dorhout Mees EJ. No effect of APD (amino hydroxypropylidene bisphosphonate) on hypercalcemia in patients with renal osteodystrophy. Bone 1990;11:15-20.
112 Davenport A, Goel S, Mackenzie JC. Treatment of hypercalcaemia with pamidronate in patients with end stage renal failure. Scand J Urol Nephrol 1993;27:447-451.
113 Frimat L, Hestin D, Trechot P, Cao Huu T, Kessler M. Severe hypercalcemia. Value of clodronate (Clastoban) in chronic renal insufficiency and hemodialysis. Apropos of 3 cases. Therapie 1993;48:496-497.
114 Ring T, Sodemann B, Nielsen C, Melsen F, Kornerup HJ. Mineralization defect but no effect on hypercalcemia during clodronate treatment in secondary hyperparathyroidism. Clin Nephrol 1995;44:209-210.
115 Torregrosa JV, Moreno A, Mas M, Ybarra J, Fuster D. Usefulness of pamidronate in severe secondary hyperparathyroidism in patients undergoing hemodialysis. Kidney Int Suppl 2003;(85):S88-90.
116 Lu KC, Yeung LK, Lin SH, Lin YF, Chu P. Acute effect of pamidronate on PTH secretion in postmenopausal hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 2003;42:1221-1227.
117 Geng Z, Monier-Faugere MC, Bauss F, Malluche HH. Short-term administration of the bisphosphonate ibandronate increases bone volume and prevents hyperparathyroid bone changes in mild experimental renal failure. Clin Nephrol 2000;54:45-53.
118 Tomat A, Gamba CA, Mandalunis P, De Grandi MC, Somoza J, Friedman S, et al. Changes in bone volume and bone resorption by olpadronate treatment in an experimental model of uremic bone disease. J Musculoskelet Neuronal Interact 2005;5:174-181.
119 Ibels LS, Alfrey AC. Effects of thyroparathyroidectomy, phosphate depletion and diphosphonate therapy on acute uraemic extra-osseous calcification in the rat. Clin Sci (Lond) 1981;61:621-626.
120 Price PA, Buckley JR, Williamson MK. The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 2001;131:2910-2915.
121 Tamura K, Suzuki Y, Hashiba H, Tamura H, Aizawa S, Kogo H. Effect of etidronate on aortic calcification and bone metabolism in calcitriol-treated rats with subtotal nephrectomy. J Pharmacol Sci 2005;99:89-94.
122 Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int 2006;70:1577-1583.
123 Monney P, Nguyen QV, Perroud H, Descombes E. Rapid improvement of calciphylaxis after intravenous pamidronate therapy in a patient with chronic renal failure. Nephrol Dial Transplant 2004;19:2130-2132.
124 Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K, et al. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis 2004;44:680-688.
125 Robinson RE, Nahata MC, Hayes JR, Batisky DL, Bates CM, Mahan JD. Effectiveness of pretreatment in decreasing adverse events associated with pamidronate in children and adolescents. Pharmacotherapy 2004;24:195-197.
126 Shaw NJ, Bishop NJ. Bisphosphonate treatment of bone disease. Arch Dis Child 2005;90:494-499.
127 Rauch F, Travers R, Munns C, Glorieux FH. Sclerotic metaphyseal lines in a child treated with pamidronate: histomorphometric analysis. J Bone Miner Res 2004;19:1191-1193.
128 Land C, Rauch F, Glorieux FH. Cyclical intravenous pamidronate treatment affects metaphyseal modeling in growing patients with osteogenesis imperfecta. J Bone Miner Res 2006;21:374-379.
129 Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med 2003;349:457-463.
130 Maalouf NM, Heller HJ, Odvina CV, Kim PJ, Sakhaee K. Bisphosphonate-induced hypocalcemia: report of 3 cases and review of literature. Endocr Pract 2006;12:48-53.
131 Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005;23:8580-8587.
132 Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 2001;12:1164-1172.
133 Banerjee D, Asif A, Striker L, Preston RA, Bourgoignie JJ, Roth D. Short-term, high-dose pamidronate-induced acute tubular necrosis: the postulated mechanisms of bisphosphonate nephrotoxicity. Am J Kidney Dis 2003;41:E18.
134 Smetana S, Michlin A, Rosenman E, Biro A, Boaz M, Katzir Z. Pamidronate-induced nephrotoxic tubular necrosis¡ªa case report. Clin Nephrol 2004;61:63-67.
Received January 23, 2007
Accepted after revision May 24, 2007
|

