|
Signal pathways in ouabain-induced
proliferation of leukemia cells
Jia-Wei Xu, Run-Ming Jin, En-Qin Li, Yan-Rong Wang, Yan Bai
Wuhan, China
Author Affiliations: Pediatric Department, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China (Xu JW, Jin RM, Li EQ, Wang YR, Bai Y)
Corresponding Author: Run-Ming Jin, Pediatric Department, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China (Tel: +86-27-85726762; Fax: +86-27-85726762; Email: jinrunm@public.wh.hb.cn)
doi:10.1007/s12519-009-0028-z
Background: Cardiotonic steroids (CTSs) can bind to Na+/K+-ATPase and activate protein kinase cascades, resulting in changes in cell proliferation, differentiation or apoptosis in a cell-specific manner. We explored the participation of ouabain-activated signaling pathways in growth regulation of leukemia cells.
Methods: Lymphocytic leukemia Jhhan cells and megakaryocytic leukemia M07e cells were incubated at different concentrations of ouabain (0, 1 and 10 nmol) for 24 hours. Cell proliferation was measured by methyl thiazolyl tetrazolium (MTT) assay. To probe the role of ouabain-induced signaling in control of cell growth, we employed Src kinase inhibitor PP2 and the MEK inhibitor PD98059, respectively. The expression of Na+/K+-ATPase ¦Á1 subunit of leukemia cells was evaluated by RT-PCR and Western blotting.
Results: One nmol and 10 nmol ouabain promoted proliferation of both Jhhan and M07e cells. Ouabain also up-regulated the expression of Na+/K+-ATPase ¦Á1 subunit. Addition of either PP2 or PD98059 blocked the effects of ouabain on cell proliferation.
Conclusion: Ouabain activates Src and ERK1/2 pathways and regulates the proliferation of leukemia cells.
Key words: cell proliferation; leukemia; Na+/K+-ATPase; ouabain; signal transduction
World J Pediatr 2009;5(2):140-145
Introduction
Cardiotonic steroids (CTSs) encompass a group of compounds that share the capacity to bind to the extracellular surface of Na+/K+-ATPase and have been used to induce positive inotropy in patients with congestive heart failure. Members of this group of compounds include plant-derived pharmaceuticals such as ouabain, and vertebrate-derived aglycone such as bufalin and marinobufagenin.[1] In 1991, Hamlyn et al[2] reported that human adrenal gland and hypothalamus could excrete endogenous ouabain.
Na+/K+-ATPase can sense low concentrations of ouabain and acts as a signal transducer.[3] Specifically, the Na+/K+-ATPase binds Src to form a functional signaling complex. Activation of this receptor complex by CTSs stimulates multiple intracellular signaling cascades that regulate cell growth in a cell-specific manner.[4] Several studies have revealed that exogenous or endogenous CTSs at ¡Ü10 nmol may stimulate cell proliferation and differentiation, and protect normal cells and some tumor cells from apoptosis.[5] But at >10 nmol they may induce cell death.[6-8] The variation in the effects of CTSs on cell proliferation or apoptosis may be due to differences in the gene expression of these cells.[5] Among the most important perturbations in cells is the over-expression and/or mutation of growth factor tyrosine kinase receptors, leading to an increased activation of down stream pathways including extracellular signal-regulated kinases (ERK)[9] and NF-¦ÊB, which protect malignant cells from apoptosis.[10]
Leukemia is a malignant disease originated from hemopoietic stem cells. With the progress in chemotherapy agents and techniques of hemopoietic stem cell transplantation, the healing rate of leukemia increases rapidly in recent years. However, the exact pathogenesis has remained uncovered yet. Here, we investigated the participation of the Na+/K+-ATPase and related signaling pathways in the growth regulation of several leukemia cell lines. Ouabain increases the proliferation of leukemia cell lines and the synthesis of ¦Á1-subunit of the Na+/K+-ATPase. These effects are sensitive to the PP2 and PD98059, inhibitors of the Na+/K+-ATPase related signaling proteins Src and ERK 1/2, respectively. On the basis of these findings it would be important to investigate whether some CTSs could be anti-leukemia drugs.
Methods
Cell culture
Lymphocytic leukemia Jhhan cells and megakaryocytic leukemia M07e cells were obtained from Professor Yang Mo of Hong Kong University. All cells were suspended in 10 ml of a solution made of IMDM (Gibco) and fetal calf serum (FCS, 10%). In addition, thrombopoietin (TPO, 10 µg/L) was added to the culture medium of the M07e cells. The cells were incubated at 37¡ãC under 5% CO2. All experiments were conducted with cells in logarithmic growth phase.
Experimental groups
In the experimental group 1, 1¡Á105/ml leukemia cells were plated in each well of a 96 multi-well plate. The cells were then incubated with ouabain at 1 nmol and 10 nmol for 24 hours, respectively. In the experimental group 2, 1¡Á105/ml leukemia cells were plated in each well of a 96 multi-well plate. They were incubated with ouabain at 1 nmol and 10 nmol for 24 hours in the presence or absence of PP2 or PD98059, inhibitors of c-Src and the extracellular regulated kinase 1/2 (ERK1/2), respectively.
Methyl thiazolyl tetrazolium (MTT) analysis
Cells cultured in a 96-well plate were serum starved for 24 hours in IMDM medium. Ouabain, PP2 and PD98059 were added as described above. Assays were done in triplicate. After treatment, 20 µl MTT (5 g/L) was added to each well and the cells were further incubated at 37¡ãC for 4 hours. Plates were centrifuged at 1000 rpm for 10 minutes and the supernatant was removed and 150 µl DMSO (Feiyi Corperation, Wuhan, China) was added into each well. The absorbance at wavelength of 492 nm was measured with a Bio-TekEXL800 Microplate Reader. The results were presented as mean ¡À SD. Leukemia cell proliferation rate was calculated by the following formula: proliferation rate (%) = (absorbance treated¨Cabsorbance control)/absorbance control ¡Á 100%. The cells without ouabain and the medium without cells were used as the blank control and negative control, respectively.
Detection of Na+/K+-ATPase ¦Á1-subunit mRNA in leukemia cells
Cells were serum starved for 24 hours in Iscove's modified Dulbecco's medium (IMDM). Ouabain, PP2 and PD98059 were added to the cells as described above. Total RNA of each group was extracted by TOYOBO RT-PCR kit (Feiyi Corperation, Wuhan, China) according to its protocol. For the reverse transcription, 1 µg (x µl) of mRNA was diluted to (11-x) µl RNase-free H2O. Thereafter, 1 ¦Ìl of oligo dT primers (10 pmol/µl) was added to the solution. For the RT reaction of each sample the following mix was made: 4 ¦Ìl of 5 ¡Á RT buffer, 2 µl of 10 mmol dNTPs, 1 µl of RNase inhibitor and 1 ¦Ìl ReverTra Ace (reverse transcriptase, Feiyi Corperation, Wuhan, China). The total volume of the mix per sample was 20 ¦Ìl. The RT procedure was as follows: 42¡ãC for 20 minutes, 99¡ãC for 5 minutes, and 4¡ãC for 5 minutes.
PCR was performed with PCR 2 ¡Á MaterMix (Tiangen Biotechnology, China) PCR reagents. The semi-quantitative PCR was done with a 20 µl reaction system composed of cDNA 3 µl, each primer (10 µmol) 0.5 µl, 2 ¡Á MasterMix 10 µl, and ddH2O 6 µl. PCR was carried out by running the following program: initial 5-minute force-denaturation (95¡ãC), 35 amplification cycles were carried out, followed by 30-second denaturation (94¡ãC), 1-minute primer annealing (60¡ãC), and 2-minute elongation (72¡ãC). The levels of target gene transcripts were quantified as the ratio of the intensity of the target gene to the intensity of ¦Â-actin. For the specific amplification of ¦Á1, forward and reverse primers were 5¡ä CTG GCT GGA GGC TGT CAT CTT CTT CAT 3¡ä and 5¡ä GTT GGG GCT CCG ATG TGT TGG GGT 3¡ä;[11] ¦Â-actin was amplified as internal control using the following primers: 5¡ä AGC CAT GTA CGT TGC TAT CC 3¡ä, 5¡ä TTG GCG TAC AGG TCT TTG 3¡ä. The sizes of PCR products were 560 bp and 498 bp. The PCR products were visualized in 1.2% agarose gel electrophoresis, the patterns were analyzed by the American alpha9900 analysis system, and results were analyzed using the software Quantity One 4.62 (Microcal Corporation, USA).
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting of isolated proteins
Cells were lysed in cold RIPA buffer (containing 1% Nonidet P40, 0.25% sodium deoxycholate, 150 mmol NaCl, 1 mmol EDTA, 1 mmol phenylmethylsulfonyl fluoride, 1 mmol sodium orthovanadate, 1 mmol NaF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 50 mmol Tris-HCl (pH 7.4) ) for 30 minutes on ice. Cell lysates were cleared by centrifugation at 13 000 rpm for 20 minutes. Supernatants were collected and assayed for protein using the Bradford method. Proteins (60 g/lane) were separated on 10% SDS-PAGE. After the electrophoretic separation, proteins were electro-blotted onto nitrocellulose membranes (Whatman Optitran, USA) at 90 V for 150 minutes. Various proteins were visualized using the enhanced chemiluminescence kit (Pierce, Rockford, IL). The ¦Á1 subunit was probed using a monoclonal anti-¦Á1 antibody and ¦Â-actin was used as a loading control. After films were developed, the density of bands were quantified using a digital documentation system and the gel image analysis software Quantity One 4.62. The target protein expression was evaluated by the relative intensity ratio of target protein/loading control (¦Â-actin).
Statistical analysis
All data were obtained from at least three repeated experiments and given as mean ¡À SD. The data were analyzed with SPSS12.0. In order to compare the data between the treated group and the control group, Student's t test was performed and significance was accepted at P<0.05.
Results
Effects of ouabain on leukemia cell lines
Leukemia cell lines Jhhan and M07e were incubated with 1 and 10 nmol ouabain for 24 hours. The MTT results showed that 1 and 10 nmol ouabain could promote proliferation of Jhhan and M07e cells in a concentration and time-dependent manner (Fig. 1).
The expression of Na+/K+-ATPase ¦Á1 subunit in Jhhan and M07e leukemia cell lines was analyzed by Western blotting. As shown in Fig. 2, Jhhan and M07e leukemia cells expressed the ¦Á1 subunit of the Na+/K+-ATPase. Exposure of both cells to ouabain increased the amount of Na+/K+-ATPase ¦Á1 subunit. A significant induction was observed when the cells were exposed to 1 nmol ouabain.
Effects of PP2 and PD98059 on ouabain-induced changes
To test whether the Src and ERK pathways were involved in ouabain-induced cell growth regulation, we determined the effects of PP2 and PD98059 on ouabain-induced cell proliferation. As shown in Fig. 3, addition of either PP2 or PD98059 blocked ouabain-induced cell growth in both Jhhan and M07e cells.
Effects of PP2 and PD98059 on ouabain-induced expressions of Na+/K+-ATPase ¦Á1 subunit
The expression of the Na+/K+-ATPase ¦Á1 subunit mRNA was quantified as the ratio of the intensity of the target gene to the intensity of ¦Â-actin. As shown in Fig. 4, the mRNA of the Na+/K+-ATPase ¦Á1 subunit of the leukemia cell lines was increased by 10 nmol ouabain. Compared with the control group (no ouabain or PP2, PD98059), the expression of the Na+/K+-ATPase ¦Á1 subunit was significantly elevated by 10 nmol ouabain (P<0.05). This induction was mostly attenuated by either PP2 or PD98059.
To confirm the above RT-PCR data, we also determined whether PP2 and PD98059 were effective in blocking ouabain-induced increases in ¦Á1 subunit protein. The same experiments were performed and the ¦Á1 protein was again measured by Western blotting. As shown in Fig. 5, the ouabain-induced increases in ¦Á1 subunit in both cell lines were substantially attenuated by either PP2 or PD98059.
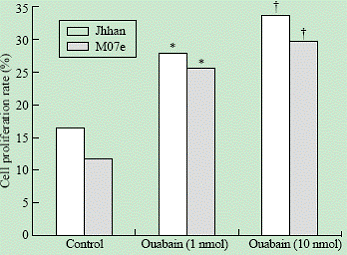
Fig. 1. Effects of ouabain on cell proliferation. Jhhan and M07e leukemia cells were exposed to 0, 1 and 10 nmol of ouabain for 24 hours and assayed for cell proliferation using MTT assay. *: P<0.05 vs control; †: P<0.05 vs control.
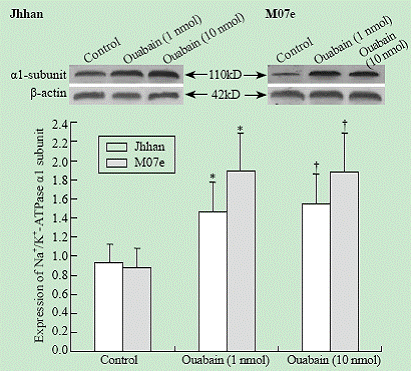
Fig. 2. Effects of ouabain on ¦Á1 expression. Cells were exposed to different concentrations of ouabain for 24 hours and the amount of ¦Á1 was probed using Western blot. Data were expressed as mean ¡À SD of three experiments. *: P<0.05 vs control; †: P<0.05 vs control.
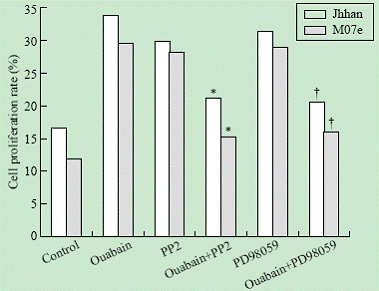
Fig. 3. Effects of PP2 and PD98059 on ouabain-induced cell proliferation. Jhhan and M07e cells were exposed to 10 nmol ouabain in the presence or absence of PP2 or PD98059 for 24 hours. Cell growth was assessed using MTT assay. Data were presented as mean ¡À SD of three separate experiments. *: P<0.05 vs control; †: P<0.05 vs control.
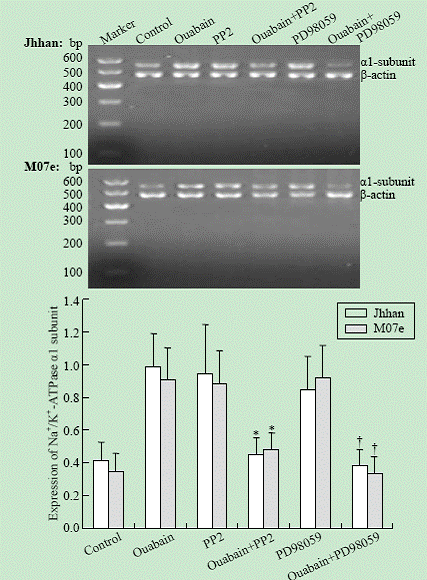
Fig. 4. Effects of PP2 and PD98059 on Na+/K+-ATPase ¦Á1 subunit mRNA expression in Jhhan and M07e cells. Cells were exposed to 10 nmol ouabain in the presence or absence of either PP2 or PD98059 for 24 hours. ¦Á1 mRNA was assessed using RT-PCR. Data were presented as mean ¡À SD of three separate experiments. *: P<0.05 vs control; †: P<0.05 vs control.
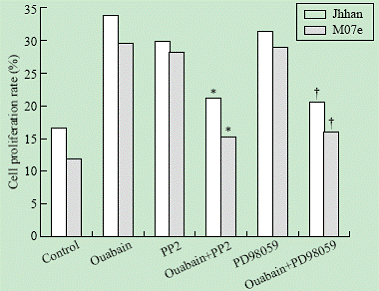
Fig. 5. Effect of PP2 and PD98059 on Na+/K+-ATPase ¦Á1 subunit expression in Jhhan and M07e cells. These experiments were done as in Fig. 4 and the amount of ¦Á1 protein was quantitated as in Fig. 2. *: P<0.05 vs control; †: P<0.05 vs control.
Discussion
Na+/K+-ATPase, a plasma membrane cation pump, is essential for maintenance of intracellular and extracellular sodium and potassium concentrations, cell volume, osmotic balance, and electrochemical gradients.[12,13] This enzyme consists of two types of subunits, designated ¦Á and ¦Â. The ¦Á subunit responsible for binding of ATP, Na+, K+, and cardiac glycosides is considered the catalytic subunit of the enzyme. Until now four ¦Á subunit variants have been identified (¦Á1-¦Á4). The ¦Á1 subunit is widely expressed in most of the human cells. The ¦Â subunit is a glycoprotein as an adhesion molecule regulating gap junction proteins, and is involved in structural and functional maturation of the holoenzyme by facilitating transport of the ¦Á subunit to the plasma membrane and maintenance of the enzyme in the lateral membrane of epithelial cells.[14-18] Na+/K+-ATPase can interact with CTSs to initiate protein-protein interaction which activates multiple signaling cascades to regulate cell proliferation, differentiation and apoptosis in a cell-specific manner.[19]
The effects of CTSs on various cancer cell lines have dual effects. Because the growth-regulatory effects of CTSs are cell-specific, we investigated the effects of low concentrations of ouabain (1 to 10 nmol) on both Jhhan and M07e leukemia cells. Our findings revealed that these leukemia cells were sensitive to the low concentrations of ouabain. One nmol ouabain was sufficient to promote the proliferation of these leukemia cells. We also found that the expression of the ¦Á1 subunit of Na+/K+-ATPase was induced by ouabain. Thus, the ¦Á1 subunit of Na+/K+-ATPase might participate in the proliferation of the leukemia cell lines induced by ouabain.
Src kinases, participating in many signaling cascades, have been implicated as a key molecule in the signaling pathways activated by ouabain/Na+/K+-ATPase interaction.[20] Co-immunoprecipitation of ¦Á1 and ¦Á2 subunits of Na+/K+-ATPase with Src is markedly increased in response to ouabain. Xie and Askari[3] proved that binding of Src to Na+/K+-ATPase could form a functional signaling complex. Specifically, the SH2 and the kinase domains of Src interact with the CD2 and CD3 domains of the Na+/K+-ATPase ¦Á1 subunit, respectively. Binding of ouabain to Na+/K+-ATPase triggers many signaling cascades that are initiated by interacting with neighboring membrane proteins. These signaling complexes send messages to intracellular organelles to regulate cell proliferation and apoptosis via the activation of the tyrosine kinase Src, transactivation of epidermal growth factor receptor, activation of mitogen-activated protein kinases [MAPKs, also termed extracellular-regulated protein kinases 1 and 2 (ERK1/2)].[20-23] We have demonstrated that PP2 (Src inhibitor) and PD98059 (MEK inhibitor) (MEK is the affector of ERK1/2) abolished the effects of ouabain on cell proliferation and on ¦Á1 expression. Surprisingly, we also found that PP2 and PD98059 promoted cell proliferation and ¦Á1 expression as ouabain did. However, it remains to be a problem that how these inhibitors regulate the ¦Á1 expression and cell growth.
Because CTSs can regulate proliferation or apoptosis in both tumor cells and normal cells, it is important for us to understand the molecular mechanism of this divergent regulation. Newman et al[24] suggested that there is a difference in the basic subunit composition of Na+/K+-ATPase that might explain the different sensitivity to CTSs between tumor and normal cells. O'Brien et al[25] found a clear preferential binding of CTSs to the ¦Á3 subunit over that of the ¦Á1 or ¦Á2 subunits. This may explain the difference of some tumor cells only express the ¦Á1 subunit but not the ¦Á3 subunit. The increased expression of ¦Á3 over ¦Á1 subunits was also noted in human colon colorectal cancer and colon adeno-carcinoma cell lines (e.g., KM12-L4, T-84, HT-29, and WiDr), whereas no significant expression of the ¦Á3 subunit protein was noted in the normal kidney and renal tissues.[26] These findings indicate that the composition of Na+/K+-ATPase ¦Á subunits may not be static within human tissues. They may shift when tissues are transformed from benign to malignant state. We presume that the different affinity of CTSs between Na+/K+-ATPase ¦Á subunits may trigger activation of various signal transduction pathways, resulting in differential regulation of cell growth. We plan to further our investigation into the specific effect of other Na+/K+-ATPase ¦Á subunits in control of leukemia cell growth.
Since Skou found that Na+/K+-ATPase has pump function in 1957, there are many studies indicating that Na+/K+-ATPase plays an important role in signal transductions. We have demonstrated that nmol concentrations of ouabain bind the Na+/K+-ATPase and activate many signaling cascades, resulting in a stimulation of cell proliferation in leukemia cell lines. Moreover, Src kinases, MEK and ERK1/2 appear to participate in the signal pathways mediated by the Na+/K+-ATPase.
Funding: None.
Ethical approval: Not needed.
Competing interest: None declared.
Contributors: Xu JW wrote the paper and all the authors approved the final version of the paper. Jin RM is the guarantor.
References
1 Dmitrieva RI, Doris PA. Cardiotonic steroids: potential endogenous sodium pump ligands with diverse function. Exp Biol Med (Maywood) 2002;227:561-569.
2 Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A 1991;88:6259-6263.
3 Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem 2002;269:2434-2439.
4 Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 2006;17:317-326.
5 Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 2007;293:C509-536.
6 Chueh SC, Guh JH, Chen J, Lai MK, Teng CM. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol 2001;166:347-353.
7 Li J, Zelenin S, Aperia A, Aizman O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-kappaB. J Am Soc Nephrol 2006;17:1848-1857.
8 Ramirez-Ortega M, Maldonado-Lagunas V, Melendez-Zajgla J, Carrillo-Hernandez JF, Pastel¨ªn-Hernandez G, Picazo-Picazo O, et al. Proliferation and apoptosis of HeLa cells induced by in vitro stimulation with digitalis. Eur J Pharmacol 2006;534:71-76.
9 Downward J. Cancer biology: signatures guide drug choice. Nature 2006;439:274-275.
10 Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 2005;5: 297-309.
11 Kulikov A, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta 2007;1768:1691-1702.
12 Lingrel JB, Kuntzweiler T. Na+,K(+)-ATPase. J Biol Chem 1994;269:19659-19662.
13 Ewart HS, Klip A. Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol 1995;269(2 Pt 1):C295-311.
14 Nesher M, Shpolansky U, Rosen H, Lichtstein D. The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sci 2007;80:2093-2107.
15 Shoshani L, Contreras RG, Rold¨¢n ML, Moreno J, L¨¢zaro A, Balda MS, et al. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol Biol Cell 2005;16:1071-1081.
16 Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci U S A 2006;103:10911-10916.
17 Caplan MJ, Palade GE, Jamieson JD. Newly synthesized Na,K-ATPase alpha-subunit has no cytosolic intermediate in MDCK cells. J Biol Chem 1986;261:2860-2865.
18 Contreras RG, Shoshani L, Flores-Maldonado C, L¨¢zaro A, Cereijido M. Relationship between Na(+),K(+)-ATPase and cell attachment. J Cell Sci 1999;112(Pt 23):4223-4232.
19 Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem 2000;275:27838-27844.
20 Kotova O, Al-Khalili L, Talia S, Hooke C, Fedorova OV, Bagrov AY, et al. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J Biol Chem 2006;281:20085-20094.
21 Manna SK, Sah NK, Newman RA, Cisneros A, Aggarwal BB. Oleandrin suppresses activation of nuclear transcription factor-kappaB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res 2000;60:3838-3847.
22 Aizman O, Uhl¨¦n P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A 2001;98:13420-13424.
23 Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J Intern Med 2007;261:44-52.
24 Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv 2008;8:36-49.
25 O'Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys 1994;310:32-39.
26 Sakai H, Suzuki T, Maeda M, Takahashi Y, Horikawa N, Minamimura T, et al. Up-regulation of Na(+),K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett 2004;563:151-154.
Received July 25, 2008 Accepted after revision December 24, 2008
|

