Insulin-like growth factors in lung development of
neonatal rats and effect of dexamethasone
andretinoic acid on their expression
Han-Chu Liu, Li-Wen Chang, Zhi-Hui Rong, Hua-Ping Zhu, Qian-Sheng Zhang, Hong-Bing Chen, Wen-Bin Li
Wuhan, China
Author Affiliations: Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China (Liu HC, Chang LW, Rong ZH, Zhu HP, Zhang QS, Chen HB, Li WB)
Corresponding Author: Han-Chu Liu, MD, Department of Pediatrics, Wuhan Children's Hospital, Wuhan 430016, China (Tel: 86-27-61335266; Email: liuhc266@hotmail.com)
Background: The lung is prone to being attacked by intrinsic/extrinsic factors and lung injury is formed. With the increased survival rate of very low birth weight, extremely low birth weight infants, respiratory failure and mortality rate caused by lung development arrest and dysplasia have increased accordingly. This study was designed to investigate the role of insulin-like growth factors (IGFs) in lung development of neonatal rats and effect of dexamethasone (DEX) and retinoic acid (RA) on their expression.
Methods: Eighty timed pregnant Sprague-Dawley rats were randomly divided into 4 groups (n=20): control group (group A), DEX treatment group 1 (group B), DEX treatment group 2 (group C), and RA treatment group (group D). Groups A, B and D were injected subcutaneously with normal saline, intraperitoneally with DEX and RA, respectively on gestational day 18-20; group C was injected subcutaneously with DEX on postnatal day 1-3. Lung tissues were collected at the following time points: gestational day 18, 20, 21 and postnatal day 1, 3, 5, 7, 10, 14, 21 respectively for histopathological examination and expression level determinations of IGF-I, IGF-II polypeptides and mRNA.
Results: The highest expression level of IGF-I in groups A and D occurred on postnatal day 5-7. There was a positive correlation between IGF-I and alveolar development. The highest expression level of IGF-I in group B was observed on postnatal day 3. The expression level of IGF-I was much higher in group B than in group A from gestational day 18 to postnatal day 3 (P<0.01); but it was significantly lower at other time points (P<0.01). The expression level of IGF-I in group C was lower but it was higher in group D than that in group A at any time points (P<0.01). The peak level of IGF-II expression occurred on gestation day 18 and reduced to trace gradually. No differences were found among all groups in the level of IGF-II expression (P>0.05). RT-PCR showed that the trends of IGF-I, -II mRNA expression in the 4 groups were in parallel with those of their polypeptides.
Conclusions: IGF plays an important role in lung development of neonatal rats and there is a close link between DEX, RA and IGF-I, -II. DEX could restrain but RA could accelerate lung development by influencing the expression of IGF-I, -II.
Key words: dexamethasone; retinoic acid; lung development; insulin-like growth factor
World J Pediatr 2007;3(1):55-60
Introduction
Modern medical technology has greatly improved the survival rate of very-low-birth-weight infants and extremely-low-birth-weight infants, but the respiratory failure caused by lung immaturity and dysplasia is still the most important factor leading to a high morbidity and mortality in the perinatal period.[1] Thus it is critical to find effective methods for the determination of lung development and its biological mechanism from basic and clinical perspectives. Recently, some researches have shown that dexamethasone (DEX), retinoic acid (RA), and insulin-like growth factor (IGF) were related to lung development and maturation,[2] but the results of different studies differed. The objective of this study was, therefore, to investigate the relationship between IGF and lung development in neonates by examining the change of IGFs in the period of lung development as well as intervening them with DEX or RA.
Methods
All procedures involving animals were carried out in accordance with the National Research Council's Guide for Care and Use of Laboratory Animals.
Experimental animals
Healthy Sprague-Dawley rats (weighing from 200 g to 250 g, 80 females and 27 males) were provided by the Center of Experimental Animals, Tongji Medical College, and kept in the laboratory for mating (female to male ratio was 3:1). The rats with sperm found in their vagina were thought as pregnant (day 0, term=22 days).[3] They were allowed food and water ad libitum.[4]
Grouping and treatment
All pregnant rats were randomly divided into 4 groups (n=20 each): control group (group A): normal saline (NS) (0.2 ml) was injected subcutaneously on gestational day 18-20; DEX 1 group (group B): DEX (0.5 mg/kg/d + NS 0.2 ml) (Binghu Shuanghe Pharmacy Company, Wuhan, China) was administered subcutaneously at the same stage as above; DEX 2 group (group C): newborn rats were subcutaneously injected with DEX (25 ¦Ìg/d) on postnatal day 1 to 3; RA group (group D): the pregnant rats were injected intraperitoneally with all trans-RA (0.5 mg/kg/d + NS 0.2 ml) (Liangfu Pharmacy Company, Shandong, China) at the same time as group A.
Collection and preparation of lung tissues
Lung tissues of fetal and neonatal rats except group C were obtained on gestational day 18, 20, 21 and postnatal day 1, 3, 5, 7, 10, 14 and 21. For tissue collection, the animals were killed with overdose of pentobarbital (50 mg/kg) injected intraperitoneally.[2] Their lungs were rapidly dissected, frozen in liquid nitrogen, and stored at -70ºC until use. For histological analysis, the lungs were perfused in situ and fixed with 4% paraformaldehyde in phosphate buffer (pH=7.2), then embedded in paraffin wax.
Lung histology, radial alveolar counts (RACs) and immunohistochemistry
Five-micrometer paraffin tissues were dissected and stained with hematoxylin and eosin. The sections were examined under a light microscope. RACs were calculated,[5] and immunohistochemistry was performed.[2] Briefly, the sections were deparaffinized in xylene, rehydrated, incubated for 30 minutes in methanol with 3% H2O2 to block endogenous peroxidase. After washing in a phosphate-buffered saline (PBS), the sections were blocked with 10% normal horse serum and incubated with mouse anti-rat IGF-I monoclonal antibody (1:25; Neomarkers, USA) or rabbit anti-rat IGF-II polyclonal antibody (1:25; Boster Bio Com, Wuhan) at 4ºC overnight. The sections were incubated with biotinylated goat anti-mouse or anti-rabbit IgG (1:200) for 30 minutes at 37ºC, and then ABC was added and staining was developed with DAB. Spatial and temporal distribution of IGF-I, -II was observed under a light microscope.
Western blotting
Lung tissue of 50 mg was lysed by homogenizing in 1 ml of protein extraction buffer (50 mmol/L Tris HCl pH=7.8, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 1% nonidet P-40, 0.1% SDS, 100 ¦Ìg/ml PMSF, 1 ¦Ìg/ml apotinin, 0.02% NaN3). Lysates were centrifuged at 12 000 r/min for 10 minutes at 4ºC and the protein samples diluted in 2¡ÁSDS-PAGE sample buffer were boiled for 5 minutes, electrophoresed on a 10% SDS-polyacrylamide gel, and transferred to the nitrocellulose membrane. Blots were blocked with 5% dried milk in PBST for 1 hour at 37ºC. Blots were incubated with rabbit antibody against rat IGF-I, IGF-II and ¦Â-actin (1:400) overnight at 4ºC and then incubated for 1 hour at room temperature with HRP-coated goat anti-rabbit IgG diluted (1:800) 5% TBST. Proteins were visualized by enhanced chemiluminescence. The relative band intensities were scanned and quantified.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from frozen lung tissues was extracted with Trizol kit (Huashun Bio Company, Shanghai) according to the manufacture's protocol. 4 ¦Ìg RNA was mixed with 1 ¦Ìl MMLV, 1 ¦Ìl of 10 mmol/L dNTP, 1 ¦Ìl of oligo (dt), 5 ¦Ìl of 5¡Ábuffer, and 0.5 ¦Ìl of Rnasine. The reaction was performed at 37ºC for 1 hour, at 95ºC for 5 minutes. cDNA was stored at -20ºC for later use. 4 ¦Ìl of the first strand cDNA reaction mixture was used for amplification in a PCR reaction system with the following components: 5 ¦Ìl of 10¡ÁPCR buffer, 1 ¦Ìl of 10 mmol/L dNTP, 2.5u TaqDNA polymerase, and 10 pmol of each primer. The reaction volume was 50 ¦Ìl. The primer sequence and PCR amplifying method were according to those described previously.[6] Two portions of each cDNA were used to amplify IGF-I/IGF-II and ¦Â-actin, respectively. The primers employed included IGF-I sense: 5'-AAGCCTACAAAGTCAGCTCG-3', antisense: 5'-GGTCTTGTTTCCTGCACTTC-3'; the PCR product size 114 base pairs (bp); IGF-II sense: 5'-ATCCCAGTGGGGAAGTCG-3', antisense: 5'-GTAGACACGTCCCTCTCG-3', product size 281 bp; ¦Â-actin sense: 5'-ATCTGGCACCACACCTTCTACAATGGCTGCG-3', antisense: 5'-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3', product size 838 bp.
Statistical analysis
All data were expressed as mean¡ÀSD and analyzed with one-way analysis of variance and q'-test by the SAS8.0 software. P<0.05 was considered statistically significant.
Results
Lung histopathological changes
On postnatal day 3, lung structure was composed of large thick-walled saccules. The number of alveoli was markedly increased on postnatal day 5 and more on day 14. Until day 21, the lung tissue contained a large quantity of alveoli with smaller airspaces and thinner walls. Interstitial cells decreased in number or disappeared. In group B, however, alveoli developed beforehand in early stage (day 3) and retarded in late stage. The structure and number of alveoli at all time points in group D were better than those in groups A, B and C (Table 1).
Table 1. Radial alveolar counts in rat lungs among the four groups (mean¡ÀSD)
|
Group |
n |
PND 3 |
PND 5 |
PND 7 |
PND 10 |
PND 14 |
PND 21 |
|
A |
20 |
4.60¡À0.11 |
6.45¡À0.15 |
7.22¡À0.21 |
9.02¡À0.19 |
10.05¡À0.16 |
10.53¡À0.31 |
|
B |
20 |
5.62¡À0.23* |
6.78¡À0.17* |
7.12¡À0.21 |
8.30¡À0.14* |
8.67¡À0.16* |
8.83¡À0.12* |
|
C |
20 |
4.90¡À0.14* |
6.62¡À0.19 |
6.95¡À0.18** |
8.03¡À0.18* |
8.47¡À0.12* |
8.78¡À0.12* |
|
D |
20 |
5.00¡À0.18* |
6.93¡À0.20* |
7.42¡À0.17 |
9.15¡À0.23 |
10.28¡À0.29 |
10.38¡À0.28** |
|
F value |
|
37.18 |
8.11 |
6.01 |
50.80 |
139.05 |
160.91 |
|
P value |
|
<0.01 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
*: P<0.05; **: P<0.01 as compared with group A. PND: postnatal day.
Immunohistochemical characteristics
Both IGF-I, -II were expressed in the developing lungs, but IGF-I mainly presented during the early alveolar stage (postnatal day 4 to 10). When the alveoli matured in groups A and D, IGF-I presented a strongly positive expression, but the expression was weakly positive in other groups or at other time points. In contrast, the peak expression of IGF-II in all groups occurred at gestational day 18, then slowly decreased to trace. After DEX was administered, the expression of IGF-II was enhanced briefly in groups B and C. However, there was no difference compared with group A.
Expression of IGF polypeptides
The expression quantity of IGF-I in group A increased gradually from gestational day 18 to a peak stage (to postnatal day 7), then decreased to trace. Compared with group A, the expression quantity of IGF-I during gestational day 20 to postnatal day 3 in group B was significantly increased (P<0.01), but significantly lowered at other time points (P<0.01). The expression was higher during postnatal days 3-5 but continuously lower at other time points in group C, and it was continuously higher in group D than in group A (P<0.01). The peak expression of IGF-II in all groups occurred at gestational day 18, then was slowly decreased to trace. But the expression during gestational day 18 to postnatal day 3 in group B was significantly higher than that in group A. No difference was found among other groups (Figs. 1, 2 and Tables 2, 3).
Table 2. Expressions of IGF-I in rat lungs among the four groups (AIGF-I/A¦Â-actin) (mean¡ÀSD)
|
Group |
GD 18 |
GD 20 |
PND 1 |
PND 3 |
PND 5 |
PND 7 |
PND 10 |
PND 21 |
|
A |
0.37¡À0.02 |
0.56¡À0.02 |
0.67¡À0.03 |
0.78¡À0.01 |
0.94¡À0.02 |
0.99¡À0.01 |
0.84¡À0.01 |
0.47¡À0.01 |
|
B |
0.36¡À0.01 |
0.59¡À0.01 |
0.74¡À0.01* |
0.86¡À0.01¦¤ |
0.81¡À0.01¦¤ |
0.77¡À0.02¦¤ |
0.62¡À0.01¦¤ |
0.35¡À0.01¦¤ |
|
C |
|
|
0.67¡À0.02 |
0.81¡À0.01¦¤ |
0.95¡À0.02 |
0.84¡À0.01¦¤ |
0.76¡À0.02¦¤ |
0.37¡À0.02¦¤ |
|
D |
0.36¡À0.01 |
0.62¡À0.02* |
0.70¡À0.02 |
0.79¡À0.02 |
1.08¡À0.03¦¤ |
0.97¡À0.02 |
0.89¡À0.02¦¤ |
0.52¡À0.02¦¤ |
|
F value |
0.31 |
14.35 |
8.16 |
47.52 |
102.30 |
207.78 |
495.20 |
134.59 |
|
P value |
>0.05 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
*: P<0.05; ¦¤: P<0.01 as compared with group A. GD: gestational day; PND: postnatal day.
Table 3. Expression of IGF-II in rat lungs among the four groups (AIGF-II/A¦Â-actin) (mean¡ÀSD)
|
Group |
GD 18 |
GD 20 |
PND 1 |
PND 3 |
PND 5 |
PND 7 |
PND 10 |
PND 21 |
|
A |
1.00¡À0.03 |
0.90¡À0.02 |
0.85¡À 0.01 |
0.73¡À 0.02 |
0.6¡À 0.03 |
0.71¡À0.03 |
0.55¡À0.02 |
0.32¡À0.01 |
|
B |
0.98¡À0.01 |
0.94¡À0.02 |
0.90¡À 0.02* |
0.83¡À 0.02¦¤ |
0.62¡À0.02 |
0.67¡À0.02 |
0.55¡À0.01 |
0.32¡À0.02 |
|
C |
|
|
0.84¡À0.01 |
0.76¡À0.01 |
0.58¡À0.01 |
0.69¡À0.02 |
0.58¡À0.02 |
0.35¡À0.03 |
|
D |
0.99¡À0.02 |
0.91¡À0.01 |
0.86¡À0.02 |
0.77¡À0.01 |
0.60¡À0.02 |
0.69¡À0.01 |
0.58¡À0.02 |
0.33¡À0.02 |
|
F value |
0.29 |
3.73 |
6.99 |
27.44 |
2.16 |
2.68 |
3.15 |
1.24 |
|
Pvalue |
>0.05 |
>0.05 |
<0.05 |
<0.01 |
>0.05 |
>0.05 |
>0.05 |
>0.05 |
*: P<0.05; ¦¤: P<0.01 as compared with group A. GD: gestational day; PND: postnatal day.
IGF-I, -II mRNA expression
RT-PCR showed that the characteristics of expression level were in parallel with those of polypeptides, i.e., the expression level of IGF-I during gestational day 20 to postnatal day 3 in group B was increased compared with group A (P<0.01), but significantly lowered at other time points (P<0.01). The expression was increased during postnatal days 3-5 but continuously lowered at other time points in group C, and was continuously higher in group D than in group A (P<0.01). The peak expression of IGF-II in all groups occurred at gestational day 18, then slowly decreased to trace. But the expression during gestational day 20 to postnatal day 3 was significantly higher in group B than in group A. No difference was found among other groups (Figs. 3, 4).
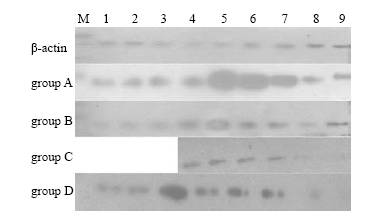
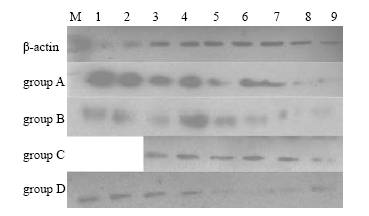
Fig. 1. The expression of IGF-I polypepties of rat lungs in the 4 groups.1-9: gestational day 18, 20, 21 and postnatal day 1, 3, 5, 7, 10, 21.Fig. 2. The expression of IGF-II polypepties of rat lungs in the 4 groups.1-9: gestational day 18, 20, 21 and postnatal day 1, 3, 5, 7, 10, 21.
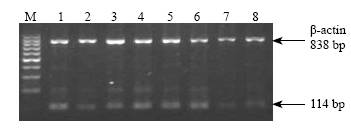
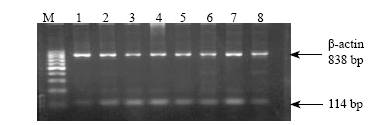
Fig. 3. The expression of IGF-I mRNA of rat lungs in group A.1-8: gestational day 18, 20, 21 and postnatal day 1, 3, 5, 7, 10, 21.Fig. 4. Expression of IGF-I mRNA of rat lungs at postnatal days 3 and 5 in different groups. M: Marker; 1-4: expression at postnatal day 3 in groups A, B, C and D respectively; 5-8: expression at postnatal day 5 in groups A, B, C and D respectively.
Discussion
To study effectively, researchers divided lung development in many mammalian species[1,7] into five stages: pseudoglandular stage, canalicular stage, saccular stage, alveolar stage, and mature stage. These stages are especially useful for comparisons between species where the timing of development differs significantly.
Role of IGFs in lung development
IGFs are important regulators of growth and differentiation of many tissues.[2] Their roles in lung development are gradually being recognized. Wallen et al[2] found that the null mutation of IGF-I, -II genes would contribute to severe retardation of lung development. Mauceri et al[8] reported that the decreasing of IGF-II level in the lung led to lung hypoplasia, whereas the decrease of IGF-I would inhibit septa subdividing. Our study further confirmed the relationship between IGF levels and lung development by determining the expression of both IGF-I, -II mRNAs and polypeptides during all stages of rat development. As expected, IGF-II mRNA and polypeptides were detected abundantly during the pseudoglandular stage, canalicular stage, saccular stage, and involved mainly in the growth and differentiation of alveolar epithelial cells and formation of airway epithelia and blood vessels. Subsequently, they declined and IGF-I expression increased markedly. The peak expression of IGF-I occurred in the early alveolar stage when epithelial cells were rapidly differentiating and forming new alveoli. The stronger the expression, the more mature the lung development. As supposed in this stage, IGF-I could promote alveolar septation, apoplosis of fibroblasts, and wall thinning of alveoli. Once alveoli matured, the expression dropped very fast. IGF-I, therefore, was thought to relate with alveolar septation and maturation.
Influence of glucocorticoid on the IGF system
DEX is commonly used in the prevention of hyaline membrane disease (HMD) and the treatment of lung dyplasia in premature infants as it can accelerate the synthesis and secretion of pulmonary surfactant.[9] However, recent studies showed that the administration of DEX pre- or post-natally could not improve the outcome of premature infants, but increase the mortality. In addition, it could inevitably inhibit alveolar septation, the thickening of alveolar wall, the enlargement of airspace, and the reduction of alveolar number. The mechnism that DEX induces lung development retardation remains unknown. Glucocorticoid is thought to stimulate micro-vessles to mature too early so that the formation of alveoli is inhibited. Recent work[10] suggested that glucocorticoid retardated lung development in 3 ways: 1) inhabiting proliferation and copy of alveolar cells during lung septation; 2) preventing septation and the alveolar walls to become thinner; and 3) interfering the formation of capillaries and reducing the synthesis of cellular DNAs by inhibiting the transform from type II alveolar epithelial cell (AECII) to AECI. Our study showed that after DEX was used pre- or post-natally, the expression of IGF-I, -II mRNA and polypeptides could be increased in a short stage so that bronchioli and blood vessles developed beforehand. With the reduction of their expression, however, the alveolarization and maturation of lungs were inhibited. The findings indicated that the inhibitory effect of DEX on lung development is related to the IGF system.[11]
Influence of RA on the IGF system
In contrast to DEX, RA played an important role in stimulating the expression of IGF mRNA and polypeptides and promoting the septation of large saccules and the maturation of alveoli.[12] Massaro et al[13] reported that the application of RA could increase the number and surface area (Sa) of alveoli and decrease their volume. After RA and DEX were used together, RA could prevent the inhibitory effect of DEX so that there was no difference in the number and Sa of alveoli between the treatment group with DEX and the control group. The present study showed that lung development in the treatment group with RA was clearly better than that in other groups, and the expressions of IGF-I mRNA and polypeptide in the group were markedly stronger than those in other groups. These suggested that the regulatory effect of RA is relevant to the IGF system.
In summary, our results indicated that the expressions of IGF-I, -II are related closely to lung development. The stronger their expressions, the better the lung development. IGF-I, -II may play their roles in different stages. The role of IGF-II is mainly played in the early period of lung development but that of IGF-I in the stage when alveolar septation is formed and matured. DEX could restrain but RA could accelerate lung development by influencing the expression of IGF-I, -II.
Funding: This study was supported by a grant from the National Natural Science Foundation of China (No. 30471824).
Ethical approval: This study was approved by the data inspectorate of China and by the Regional Committee for Medical Research Ethics.
Competing interest: None declared.
Contributors: Liu HC wrote the first draft of this paper. All authors contributed to the intellectual content and approved the final version. Chang LW is the supervisor and the guarantor.
References
1 McMurtry IF. Introduction: pre- and postnatal lung development, maturation, and plasticity. Am J Physiol Lung Cell Mol Physiol 2002;282:L341-344.
2 Wallen LD, Myint W, Nygard K, Shimasaki S, Clemmons DR, Han VK. Cellular distribution of insulin-like growth factor binding protein mRNAs and peptides during rat lung development. J Endocrinol 1997;155:313-327.
3 Chang LW, Rong ZH, Zhang QS. Effect of retinoic acid on lung injury in hyperoxia-exposed newborn rats. J Huazhong Univ Sci Technol Med Sci 2003;23:71-74.
4 Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman N, Ozkal S, et al. Effects of erythropoietin on hyperoxic lung injury in neonatal rats. Pediatr Res 2005;58:38-41.
5 Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600-607.
6 Oue T, Taira Y, Shima H, Miyazaki E, Puri P. Effect of antenatal glucocorticoid administration on insulin-like growth factor I and II levels in hypoplastic lung in nitrofen-induced congenital diaphragmatic hernia in rats. Pediatr Surg Int 1999;15:175-179.
7 Wallen LD, Han VK. Spatial and temporal distribution of insulin-like growth factors I and II during development of rat lung. Am J Physiol 1994;267(5 Pt 1):L531-542.
8 Mauceri HJ, Becker KB, Conway S. The influence of ethanol exposure on insulin-like growth factor (IGF) type II receptors in fetal rat tissues. Life Sci 1996;59:51-60.
9 Luyet C, Burri PH, Schittny JC. Suppression of cell proliferation and programmed cell death by dexamethasone during postnatal lung development. Am J Physiol Lung Cell Mol Physiol 2002;282:L477-483.
10 Massaro D, massaro GD. Invited review: pulmonary alveoli: formation, the "call for oxygen", and other regulators. Am J Physiol Lung Cell Mol Physiol 2002;282:L345-358.
11 Bloomfield FH, Knight DB, Breier BH, Harding JE. Growth restriction in dexamethasone-treated preterm infants may be mediated by reduced IGF-I and IGFBP-3 plasma concentrations. Clin Endocrinol (Oxf) 2001;54:235-242.
12 Chetty A, Manzo N, Waxman AB, Nielsen HC. Modulation of IGF-binding protein-2 and -3 in hyperoxic injury in developing rat lung. Pediatr Res 2005;58:222-228.
13 Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol 1996;270(2 Pt 1):L305-310.
Received August 31, 2006 Accepted after revision December 4, 2006

