| |
Neonatal severe anemia caused by
parvovirus B19 infection
Fu-Gen Wu, Yuan-Hai Zhang, Hong Chen, Feng Lin, Jian-Min Zhang
Wenling, China
Author Affiliations: Department of Pediatrics, Wenzhou Medical College Affiliated Wenling Hospital, Wenling 317500, China (Wu FG, Chen H, Lin F, Zhang JM); Department of Pediatrics, Yuying Children's Hospital of Wenzhou Medical College, Wenzhou 325027, China (Zhang YH).
Corresponding Author: Fu-Gen Wu, Department of Pediatrics, Wenzhou Medical College Affiliated Wenling Hospital, Wenling 317500, China (Tel: 86-576-86206252; Email: docwufg@sina.com.cn)
Background: Human parvovirus B19 may cross the placenta and result in fetal infection, morbidity and death. We present here a case of newborn infant with severe anemia who died from human parvovirus B19 infection.
Methods: Analysis was made based on the clinical presentations. According to the case history, physical and laboratory findings, neonatal severe anemia induced by parvovirus B19 infection was suggested; autopsy and pathological examination were performed on the infant subsequently.
Results: The hemoglobin level was significantly low at 19 g/L. The infant died after emergency treatment and was subjected to autopsy. The diagnostic nuclear inclusion for parvovirus B19 infection were observed in the marrow, liver, spleen, and placenta under the light microscope.
Conclusion: Clinical presentation and pathological studies all indicated that the infant was infected with parvovirus B19.
Key words: human parvovirus B19; neonate; infection; anemia
World J Pediatr 2007;3(3):232-235
Introduction
Parvovirus B19 (B19 virus) is the smallest single strand linear DNA virus in animal viruses, which is the only strain of parvovirus that is pathogenic in humans. Since Anderson and colleagues identified B19 as the cause of erythema infection in 1983,[1] parvovirus B19 infection has received more and more attention and has become one of the hot topics in medical research. In the present report, we described a case of newborn infant with severe anemia caused by parvovirus B19 infection, who was admitted to our hospital in 2002.
Case report
A 2500 g female infant was born to a 27-year-old mother, Gravida 3 Para 2 by emergency cesarean section at 39-week gestation because of intrauterine distress. With Apgar score of 2 at 1 minute, the infant received resuscitation with mask ventilation, intubation, and cardiac compression in the delivery room. Half an hour later, Apgar score was 4, and she was transferred to neonatal intensive care unit (NICU) on account of low reaction, systematic pallor and weak respiration. The placenta was complete but pale and edematous; the umbilical cord was also edematous and not around the neck. Amniotic fluid was clear.
The mother's first gestation failed in stillbirth with unknown reasons, the second one was spontaneously aborted after pregnancy for 2 months, and the third (i.e., the fetus mentioned in the paper) was conceived before normal menstrual onset. The mother complained of wheal-like skin rash during the onset of pregnancy but denied history of raising cat or dog. There was no significant family history or relevant inherited diseases.
Physical examination on the newborn showed systematic pallor and low body temperature; the infant was in coma and with no reactions even with tracheal intubation. No adenopathy, jaundice or skin rash was found. The newborn had a head circumference of 34 cm and body length of 49 cm. Moist rales were found in both lungs; heart rate was 98/min. Heart sounds were dull and low-pitched, without significant murmurs. The abdominal wall was tender. Liver was palpated about 3.0 cm under the right costal margin and 4.0 cm under the xiphoid. The spleen was untouched. Shifting dullness was negative and bladder region was dull on percussion.
The laboratory findings showed significantly low levels of hemoglobin (19 g/L; normal range: 170-200 g/L) and red blood cells (0.46 ¡Á 1012/L; normal range: 6.0-7.0 ¡Á 1012/L), but high levels of the total white blood cells (56 ¡Á 109/L; normal range: 15.0-20.0 ¡Á109/L) and blood sugar (13 mmol/L; normal range: 1.5-6 mmol/L). The fetal hemoglobin (hemoglobin F) in maternal blood was less than 3%. Red blood cell acid elution test was negative. The newborn's blood type was the same as the maternal one of type "O" and Rh positive. So the neonatal hemolytic anemia caused by blood type incompatibility was excluded.
The condition of the newborn was severe even after receiving intubation, mechanical ventilation, fluid replacement, and treatment with vasoactive agents. Lots of transparent fluid was withdrawn from the cannula. After only 2 hours of hospital stay, the newborn died with cardiorespiratory arrest during the emergency treatment. She was then subjected to autopsy.
Anatomic diagnosis and pertinent findings at postmortem were as follows. Bleeding points on plasma membrane in the lungs were found. Both lungs were edematous, the liver and both kidneys were enlarged and pale. Congestive heart failure was indicated with generalized visceral enlargement and pallor. Pleural cavities, abdominal cavity and pericardial cavity were filled with yellow fluid. Lienal acinus decreased and poorly developed.
Microscopically, placental capillaries were filled with intranuclear inclusions by hematoxylin and eosin staining. The nuclei of cells had a marginated, irregular band of darkly stained chromatin, and the center of the nucleus was lighter in color and appeared to have a smooth, glassy texture. Pulmonary hyaline membrane was formed on the surface of pulmonary alveoli and lung vascular spaces were filled with nucleated red blood cells and typical intranuclear inclusions. The inclusion bodies were also seen within intermediate erythroblast nucleus of medullary cavitas, hematopoietic sinus, splenic sinus, and extramedullary hematopoiesis focus (Figs. 1-4).
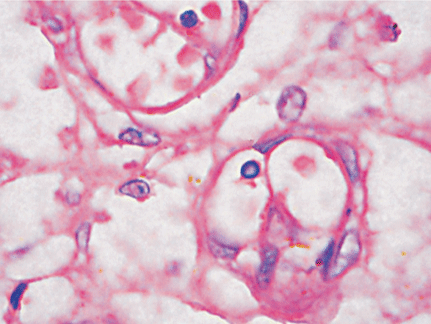 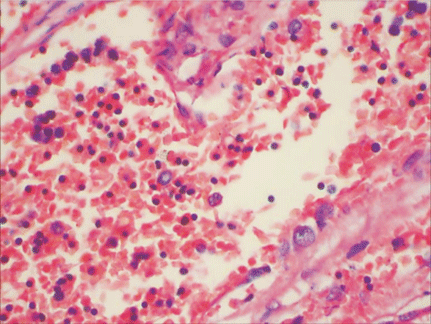
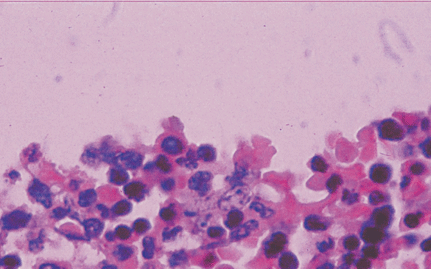 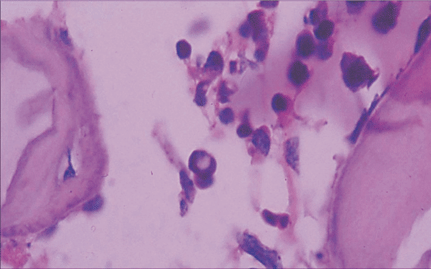
ig. 1. Placenta capillaries filled with intranuclear inclusions. The nuclei have a marginated, slightly irregular band of darkly staining chromatin, and the center of the nucleus is lighter in color and appears to have a smooth, glassy texture with hematoxylin and eosin staining (original magnification ¡Á 400).Fig. 2. Lung vascular spaces filled with nucleated red blood cells and typical intranuclear inclusions shown with ematoxylin and eosin staining (original magnification ¡Á 200).Fig. 3. Characteristic giant pronormoblasts observed in splenic sinus and extramedullary hematopiesis (original magnification ¡Á 400).Fig. 4. Characteristic giant pronormoblasts in the bone marrow (original magnification ¡Á 400).
The characteristics and specific findings on histological examination from the liver, spleen, sternum and placental tissue indicated intrauterine B19 infection. The B19 infection resulted in the failure of hematopoiesis and clinical presentations of severe anemia, pulmonary edema and hyaline membrane disease.
Discussion
Parvovirus B19 could infect fetus via the placenta, or lead to fetal hydrops, congenital anemia, abortion, immature labor, stillbirth, or congenital malformations.[2,3]
Parvovirus B19 is a member of the erythrovirus genus parvoviridae family[4] which includes similar simian viruses[5] propagating best in erythroid progenitor cells. Infection with B19 virus is global,[6] and infection rates are similar in the United States, Europe, and Asia. The virus is spread by respiratory droplets, household contacts, blood products and maternal-neonatal transmission. The infection rate of B19 is varied with region, season, age, vocation and immune state, which are commonly seen in children and pregnant women. The reported positive rates of serum B19 IgG antibody in pregnant women or those at child-bearing period are 16%-81%, those of IgM antibody in recent B19 virus infection 1.0%-5.0%, and those of transplacental transmission 24%-33%. The overall risk of abnormal outcome is approximately 5% to 10%.[7-9] The infection is occupation-dependent and age-dependent; the infection rates in the populations who are day-care workers in kindergartens, teachers in primary schools, and medical workers are significantly higher than those in others. The infection risk will increase by 5 times for those who have a daily contact with children.[10] Because of the high infection rates and high risks, these populations should be detected, especially for the pregnant vocational women.
B19 virus infection in fetus can cause fetal hydrops and congenital anemia, accounting for 10%-20% of all nonimmunologic hydrops fetalis cases.[11] The pathogenesis of the anemia induced by persistent B19 viral infection is mainly associated with the damage of erythroid cells in hematopoietic tissues. The only known natural host cell of parvovirus B19 is the human erythroid progenitor.[6] This extraordinary tropism was first demonstrated in tissue culture, where a small amount of parvovirus B19 markedly inhibited colony formation by early and especially by late erythroid progenitors without affecting myeloid colony formation.[6] Schwarz et al[12] in 1991 confirmed by the immunohistochemical method that the target cell of B19 virus is nucleated red blood cells. Soon after, with the molecular cloning recombination technique, Brown et al[13] also identified that the cell receptor of B19 virus was P antigen (globoside), revealing the biological pathogenic basis of B19 virus. P antigens can be seen not only on the surface of erythroid precursors and red cells, but also on some megacaryocytes, endothelial cells, some types of placental cells, liver and myocardial cells of the fetus. Rare persons, whose erythrocytes lack P antigen, are not susceptible to infection with parvovirus B19.[14] The secondary immune response of body contributes to the diversified clinical manifestations.[7] Because of the immature fetal immune system, especially before the 20th week of pregnancy, B19 virus invading into body will multiply in a large quantity. Fetal damage appears to be similar to that in patients with aplastic crisis, in whom erythrocytes have a reduced life span. Intrauterine infection is persistent and characterized by severe anemia, hypoxia, high-output cardiac failure, and death. In addition, B19 virus can invade the liver, resulting in hepatic functional disorder or even hepatitis and hepatic fibrosis; it can also invade cardiac muscle, causing myocarditis. The basis of persistent parvovirus infection is a defect in immunoglobulin production.[6]
Laboratory tests for the diagnosis of B19 infection are not available yet. Anti-B19 IgM and IgG antibodies detection is a way to diagnose B19 infection in an affected fetus. Fetal hydrops or anemia along with specific IgM in maternal serum provides strong presumptive evidence of intrauterine B19 infection, and collection of fetal specimens may involve undue additional risk to the fetus without a significant increase of the diagnostic yield.[15] DNA detection is necessary for those who suffer from immune deficiency and persistent infection because in whom, antibody production is absent or minimal. Hybridization of nucleic acid and polymerase chain reaction (PCR) techniques can be applied to detect maternal blood, bone marrow, cord blood and embryonic tissue, which are widely used and known for their high specificity, by which the infection of B19 in specific target cells can be detected rapidly and accurately.[7,15]
Routine histological studies can play an important role in the diagnosis of B19 infection.[15] Finding the characteristic intranuclear inclusions in nucleated erythroid cells in the formalin-fixed placenta or fetal tissues stained with hematoxylin and eosin provides strong presumptive evidence of intrauterine B19 infection. With hematoxylin and eosin staining, the nuclei have a marginated, slightly irregular band of darkly stained chromatin, and the center of the nucleus is lighter in color and appears to have a smooth, glassy texture. This is called diagnostic nuclear inclusion.[15-17] The cytopathic effect of infection of erythroid progenitor cells with B19 virus is manifested as that of giant pronormoblasts (lantern cells).[7] Giant pronormoblasts are the precursors of erythroid cells with a diameter of 25 to 32 ¦Ìm, large eosinophilic nuclear inclusion bodies, and cytoplasmic vaculization, and occasionally, "dog-ear" projections.[7,18] Affected cells are observed within the lumen of vessels in any organ, or in the parenchyma in sites of extramedullary hematopoiesis, chiefly in the liver, spleen and placental tissue.[15]
In this patient, the characteristical and specific findings of giant pronormoblasts on histological examination from the liver, spleen, sternum and placental tissue were consistent with intrauterine B19 infection. Detection of B19-IgM and B19-DNA in maternal blood, cord blood and neonatal blood was not performed because of the infant's critical condition.
In most cases, parvovirus infection does not require specific therapy.[2,6] No methods are available for prediction of the prognosis of the infected fetus. For pregnant woman who has been diagnosed to have B19 infection, whose serum ¦Á-fetoprotein level should be monitored and the fetal condition should be examined with ultrasonography. Follow-up should be continued for 12 weeks after mother's final diagnosis of virus infection.[19] For persistent B19 infection, effective therapy consists of immunoglobulin infusion at a 5- or 10-day course at a dose of 0.4 g per kilogram of body weight.[6] Fetal hydrops may resolve spontaneously. Intrauterine blood transfusion has been used successfully but the risk is high. Vaccine phase I trials have shown promising results, and Phase II trials are planning.[20] Effective vaccines are available for animal parvoviruses, but their safety is still under evaluation.
Acknowledgements
The authors wish to thank Professor Mei-Yue Sun for her sincere advice on the article writing.
Funding: None.
Ethical approval: This study was approved by the Data Inspectorate of China and by the Regional Committee for Medical Research Ethics.
Competing interest: None declared.
Contributors: Wu FG wrote the first draft of this paper. All authors contributed to the intellectual content and approved the final version.
References
1 Anderson MJ, Jones SE, Fisher-Hoch SP, Lewis E, Hall SM, Bartlett CL, et al. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet 1983;1:1378
2 Behram RE, Kliegman RM, Jenson HB. Nelson textbook of pediatrics, 16th ed. Philadephia: WB Saunders Company, 2001: 964-966.
3 Brown T, Anand A, Ritchie LD, Clewley JP, Reid TM. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet 1984;2:1033-1044.
4 Brown KE. Variants of B19. Dev Biol (Basel) 2004;118:71-77.
5 Brown KE, Young NS. The simian parvoviruses. Rev Med Virol 1997;7:211-218.
6 Young NS, Brown KV. Parvovirus B19. N Engl J Med 2004; 350:586-597.
7 Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev 2002;15:485-505.
8 Candotti D, Danso K, Parsyan A, Dompreh A, Allain JP. Maternal-fetal transmission of human parvovirus B19 genotype 3. J Infect Dis 2006;194:608-611
9 Ramirez MM, Mastrobattista JM. Diagnosis and management of human parvovirus B19 infection. Clin Perinatol 2005;32: 697-704.
10 Adler SP, Manganello AM, Koch WC, Hempfling SH, Best AM. Risk of human parvovirus B19 infections among school and hospital employees during endemic periods. J Infect Dis 1993;168:361-368
11 Jordan JA. Identification of human parvovirus B19 infection in idiopathic nonimmune hydrops fetalis. Am J Obstet Gynecol 1996;174(1 Pt 1):37-42.
12 Schwarz TF, Nerlich A, Hottentrager B. Parvovirus B19 infection of the fetus. Histology and in situ hybridization. Am J Clin Pathol 1991;96:121-126.
13 Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 1993;262(5130): 114-117.
14 Brown KE, Hibbs JR, Gallinella G, Anderson SM, Lehman ED, McCarthy P, et al. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N Engl J Med 1994;330:1192-1196.
15 Remington JS, Klein J, Baker C, Wilson C. Infectious Diseases of the Fetus and the Newborn Infant, 6th ed. Philadelphia: Saunders, 2005: 779-801.
16 Kurman RJ. Blaustein's Pathology of the Female Genital Tract, 5th ed. New York: Springer, 2002: 1128.
17 Fox H, Wells M. Haines and Taylor Obstetrical and Gynaecological Pathology, 5th ed. New York: Churchill Livingstone, 2002: 1317.
18 Caul EO, Usher MJ, Burton PA. Intrauterine infection with human parvovirus B19: a light and electron microscopy study. J Med Virol 1988;24:55-66
19 Crane J. Parvovirus B19 infection in pregnancy. J Obstet Gynaecol Can 2002;24:727-743.
20 Ballou WR, Reed JL, Noble W, Young NS, Koenig S. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C 1. J Infect Dis 2003;187:675-678.
Received August 18, 2006 Accepted after revision May 16, 2007
|

