|
The relationship between expressions of the
laminin gene and RET gene in Hirschsprung's
disease
Ai-Wu Li, Wen-Tong Zhang, Rong Wang, Jin-Bo Feng, Yi Ruan
Jinan, China
Author Affiliations: Department of Pediatric Surgery, Qilu Hospital of Shandong University, Jinan 250012, China (Li AW, Zhang WT); Key Lab of Cardiovascular Functional and Remodeling Research of Ministry of Education and Health, China (Wang R, Feng JB); Department of Microbiology of Medical Collage of Shandong University, China (Ruan Y)
Corresponding Author: Wen-Tong Zhang, Department of Pediatric Surgery, Qilu Hospital of Shandong University, Jinan 250012, China (Tel: 86-531-82169400; Email: liaiwuxie@yahoo.com.cn)
Background: The cause of Hirschsprung's disease (HD) remains unclear, but currently there are two theories: the mutation of the RET gene and the change of enteric microenvironment. This study was undertaken to elucidate the cause of HD by assessing the expression of laminin (LN), laminin gene, and the RET gene in the aganglionic segment, transitional zone and normal segment of the colon in patients with HD.
Methods: Specimens of the aganglionic segment, transitional zone, and normal segment of the colon from 27 cases of HD were stained immunohistologically by a PV 9000 polymer detection system. Photos were taken by the RS image system, and the staining area of each image was calculated by a JD 801 image analysis system. The qualitative expressions of the laminin gene and RET gene of these three segments in the 27 cases were detected by reverse transcription-polymerase chain reaction (RT-PCR), and the difference of the expressions was shown by the alpha 9900 image analysis system. The quantitative expressions of the laminin gene and RET gene in the three segments were detected by real-time quantitative PCR, and the difference of the expression was shown by SDS software.
Results: The laminin and laminin gene were expressed in all the three segments. The expression was higher in the aganglionic segment than in the dilated segment, and the expression decreased stepwisely from the aganglionic segment to the normal segment, while the expression of the RET gene was opposite, showing an increased segmenting from the aganglionic segment to the normal segment. The correlation between the expressions of the two genes was negatively correlated.
Conclusions: The highly increased expression of LN in the aganglionic segment may cause early differentiation, early maturation and premature ecesis of enteric nervous cells. The change of the microenvironment of colon wall may be the cause of HD. The negative correlation between the expression of the two genes may be closely related to the occurrence of HD.
Key words: gene expression; Hirschsprung's disease; laminin; RET
World J Pediatr 2008;4(2):135-139
Introduction
The cause of Hirschsprung's disease (HD) remains obscure, but two theories are available: the mutation of the RET gene and the change of enteric microenvironment.[1] The main component of the extracellular matrix is laminin (LN) which greatly contributes to the differentiation, maturation and ecesis of enteric nerve cells.[2] The change of the expression of LN may cause the abnormal distribution of enteric nerve cells.
This study aimed to elucidate the cause of HD by assessing the expressions of the laminin, laminin gene, RET gene and the correlation between the laminin gene and RET gene in the aganglionic, transitional and normal segments of the colon of HD patients using immunohistological staining, reverse transcription polymerase chain reaction (RT-PCR) and real-time quantitative PCR.
Methods
Materials
Specimens were obtained from 27 patients with pathologically confirmed HD pre- or post-operatively at the Department of Pediatric Surgery, Qilu Hospital of Shandong University in 2004. The narrow segment was taken as the aganglionic segment, the transitional segment or the distal end of the dilated segment with normal ganglion cells pathologically confirmed as the ganglionic segment, and the proximal end of the dilated segment as the normal segment. Colonic tissue (200 mg) was taken from each of the three segments respectively and stored at -80ºC. Similar tissues from the three segments were fixed in 10% neutral-formalin and embeded with paraffin.
Mouse anti-laminin monoclonal antibody, PV-9000 polymer detection system for immunohistological staining, and ZLI-9033 DAB kit were purchased from American Golden Bridge International Company. RT-PCR kit was purchased from American Promega Company. RT-PCR instrument was Tgradient 96, and electrophoresis apparatus was Powerpac. The mixed SYBR green kit of ABI Company was made for real-time quantitative PCR with an ABI Prism 7000 instrument.
Contrivance and synthesis of primers
Human ¦Â-actin was taken as an internal control with the production of 317 base pair (bp). There were three side chains in laminin. A pair of primers was constructed by the cDNA Chi sequence of the alpha 1 chain of laminin. Its product was 186 bp. Another pair of primers was constructed by the cDNA Chi sequence of the RET gene. Its production was 101 bp. These three pairs of primers were synthesized and purified by Shanghai Biological Engineering Company. The following were the three pairs of primers: Human ¦Â-actin, sense P1: 5'ATCATGTTTGAGACCTTCAACA 3', antisense P2: 5'CATCTCTTGCTCGAAGTCCA3'; Human laminin alpha 1, sense P1: 5'GATTGGTGATGCCGTTCTTT3', antisense P2: 5'TTCTTTTGCAGGTTGTCCGT3'; Human RET, sense P1: 5'CCAGGGTCGGATTCCAGTTA3', antisense P2: 5'CCCACAGCAGGACACCAAAA3'.
Methods
Immunohistological staining
The samples were cut into 5 ¦Ìm thick sections, embedded, deparaffinized, hydrated, and incubated for 10 minutes by 3% H2O2 which had been deionized so as to block the endogenous peroxydase. The antigen of LN was repaired by diastase vera. Then, mouse anti-laminin was added and incubated for 12 hours at 4ºC, and washed by PBS. Polymer helper was dropped and incubated for 20 minutes at 37ºC, and washed by PBS. Polyperoxidase-anti-mouse was dropped in and incubated for 20 minutes at 37ºC, washed by PBS, and then stained by DAB. The negative control was made by PBS instead of mouse anti-laminin. Brown and yellow deposition represented a positive reaction. Density and distribution were observed under a light microscope. Images were taken by the RS image system, and the scale in these photos was 50 ¦Ìm. The area of staining in each image was calculated by multilayer staining method of the JD 801 image analysis system.
PCR
The con-RNA of each sample was extracted respectively according to the description of the RT-PCR kit. The optical density value (OD value) of RNA was determined and calculated by the equation, A260/A280. Then cDNA was synthesized by reverse transcription in 20 ¦Ìl reaction buffer containg con-RNA 1 ¦Ìg, M-MLV 200 U/¦Ìl, primer 10 ¦Ìg/L. The reaction was continued for 1 hour at 37ºC, and then stretched for 10 minutes at 95ºC. The reactant was tacho-centrifuged.
The semi-quantitative PCR was done with a 50 ¦Ìl reaction system composed of cDNA 0.1 ¦Ìg/¦Ìl, each primer 10 ¦Ìg/L, Taq-polymerase 1 U/¦Ìl, etc. The PCR condition included force-denaturation at 94ºC for 5 minutes, denaturation at 94ºC for 1 minute, renaturation at 58ºC for 1 minute, elongation at 72ºC for 1 minute, totally for 35 cycles. Then it stretched at 72ºC for 7 minutes. The products were visualized in 1.5% agarose gel electrophoresis, the patterns were analyzed by the American alpha9900 analysis system, and the quantity of genes was observed. The calculated relative coefficient was equal to the intensity of the goal gene as divided by the expressive intension of ¦Â-actin.
At last, the real-time quantitative PCR was done with a 50 ¦Ìl reaction system composed of 2 ¡Á SYBR green mixed buffer 25 ¦Ìl. The reaction condition was: force-degeneration, 50ºC 10 seconds; degeneration, 95ºC 10 minutes; renaturation, 95ºC 15 seconds; elongation, 60ºC 1 minute; this course was circulated for 40 times. After the termination of PCR, the production was analyzed by the 7000-SDS system automatically. Each amplification curve of reaction and CT value was observed. Each sample was duplicated for 6 times. The average CT value was the extreme CT value of the sample.
The expression difference of the gene was calculated by the 2-¦¤¦¤CT method,[3] ¦¤CT = CT value of the goal gene ¨C CT value of the ¦Â-actin. The normal segments were taken as the control group, ¦¤¦¤CT = ¦¤CT of the goal gene group ¨C ¦¤CT of the control gene group. The expression of normal segment was taken as 1, 2-¦¤¦¤CT that would be the multiple genes of the goal segment compared with the normal segment.
Statistical analysis
All the data were analyzed with SPSS 11.5. The area of positive staining of the three segments was compared by one-way ANOVA; the relative coefficient of the gene expression was also compared by one-way ANOVA. The average CT value of each segment of the 27 samples was calculated by SPSS for Windows 11.5 software, as was expressed by means ¡À SD. The correlation between the expressions of the laminin alpha 1 and RET gene was calculated by Pearson's correlation. A P value less than 0.05 was considered statistically significant.
Results
Immunohistological staining
Brown yellow deposition was a positive reaction shown by immunohistological staining. Positive reaction mainly appeared in the basement membrane of mucosa, submucosa and inner circular muscle layer. The density of staining showed a decrease from submucosa toward outside circular muscle layer. Brown yellow deposition in the normal segment was punctiform, whereas that in the aganglionic segment was far more abundant and widespread in submucosa and was reticulodromous in the circular muscle layer. Laminin immunoreactivity showed a regional decrease from the aganglionic segment to the normal segment with significant deviation (Table 1, Fig. 1).
RT-PCR
The OD value of RNA calculated by A260/A280 varied from 1.8 to 2.0. The quantity of laminin alpha 1mRNA was reduced from the aganglionic segment to the normal segment (by American alpha9900 analysis system), while the quantity of RETmRNA was increased from the aganglionic segment to the normal segment (Fig. 2). The relative coefficient was significantly different (Table 2).
Real-time PCR
In the course of real-time quantitative PCR, the amplification curve was shown by fluorescent threshold and cycle, and a fair reproducibility of each sample and basically coincident efficacy amplification were demonstrated. In this study, the expression of laminin alpha 1 in the aganglionic segment was twice that in the normal segment by the real-time quantitative PCR, and laminin alpha 1 mRNA reduced gradually in the aganglionic segment to the normal segment (Table 3). The expression of RET was reduced significantly in the aganglionic segment than in the normal segment by the real-time quantitative PCR, and RET mRNA increased gradually in the aganglionic segment to the normal segment (Table 4).
The correlation between the expression of the LN gene and RET gene was calculated by bivariate-Pearson's correlation. A significant inverse correlation was shown with a correlation coefficient of -0.638 (P<0.001). In the aganglionic segment, laminin alpha 1 mRNA increased but RETmRNA decreased.
Table 1. The distribution of laminin in three segments (percentage of staining area to whole area %, means ¡À SD)
|
Content |
Aganglionic segment |
Ganglionic segment |
Normal segment |
|
LN |
16.33¡À5.15*† |
10.85¡À3.67 |
8.47¡À3.43 |
*: P<0.05 vs normal segment; †: P<0.05 vs ganglionic segment.
Table 2. The relative coefficient of the laminin gene and RET gene in the three segments (means ¡À SD)
|
Gene |
Samples |
Aganglionic segment |
Ganglionic segment |
Normal segment |
|
Laminin alpha 1 mRNA |
27 |
0.73¡À0.18*† |
0.39¡À0.15* |
0.15¡À0.12 |
|
RET mRNA |
27 |
0.07¡À0.05*† |
0.33¡À0.14* |
0.79¡À0.19 |
*: P<0.05, vs normal segment; †: P<0.05, vs ganglionic segment.
Table 3. The relative quantity of laminin alpha 1 mRNA in three segments
|
Segment
|
Laminin alpha 1 average
CT value |
¦Â-actin average
CT value |
¦¤CT
|
¦¤¦¤CT
|
Times of the gene
(compared to normal segment) |
|
Normal |
30.54¡À4.90 |
29.87¡À5.33 |
0.67 |
0 |
1 |
|
Ganglionic |
31.72¡À3.98 |
31.20¡À5.57 |
0.52 |
-0.15 |
1.11 |
|
Aganglionic |
30.18¡À2.65 |
30.49¡À5.81 |
-0.31 |
-0.98 |
1.97 |
Table 4. The relative quantity of RET mRNA in three segments
|
Segment
|
RET
CT value |
¦Â-actin average
CT value |
¦¤CT
|
¦¤¦¤CT
|
Times of the gene
(compared to normal segment) |
|
Normal |
28.46¡À6.30 |
29.87¡À5.33 |
-1.63 |
0 |
1 |
|
Ganglionic |
30.85¡À4.61 |
31.20¡À5.57 |
-0.35 |
1.28 |
0.41 |
|
Aganglionic |
32.64¡À3.77 |
30.49¡À5.81 |
2.15 |
3.78 |
0.07 |
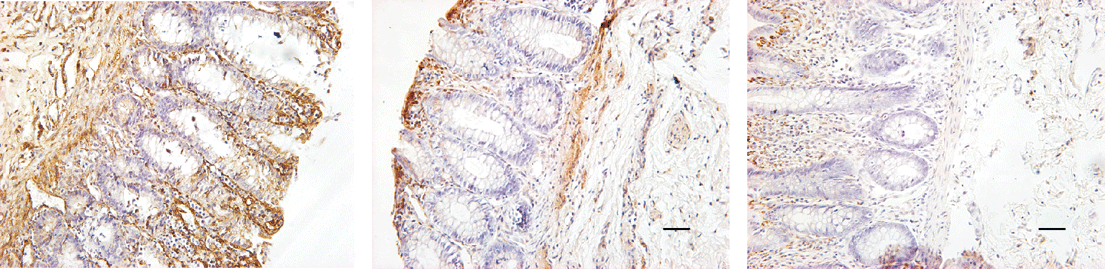
Fig. 1. Immunohistochemical staining of laminin (bar=50 ¦Ìm). A: aganglionic segment (The staining of laminin immunoreactivity was hyperchromic and lamellar); B: transitional segment (The staining of laminin immunoreactivity was linear-like); C: normal segment (The stain of laminin immunoreactivity was faintly colored and punctifo
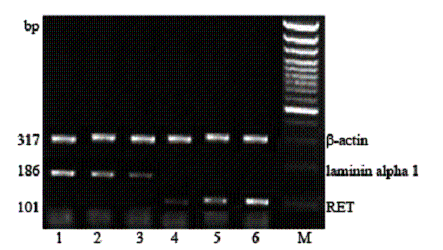
Fig. 2. The expression of laminin alpha 1 mRNA, RET mRNA, ¦Â-actin mRNA by RT-PCR. Lanes 1, 2, 3: laminin alpha 1 mRNA of the aganglionic segment, ganglionic segment and normal segment, respectively. Lanes 4, 5, 6: RET mRNA of the aganglionic segment, ganglionic segment and normal segment, respectively.
Discussion
The enteric nervous system (ENS) is originated from the neural crest cell. When neural crest cell comes into the intestinal tract, it migrates, generates and differentiates into ganglion cells and glial cells, then develops into the Auerbach nervous plexus and Meissner nervous plexus. This migration course is influenced by enteric microenvironment.[4] The differentiation of neural crest cells has more potentiality. The migration and differentiation have not been proved previously, and the enteric microenvironment might be the main causative factor. Aganglionosis is due to the maldevelopment of ENS.[5]
LN can adhere to its specific receptor on the surface of cell membrane. It makes the growing tip discriminate and cohere to the cell matrix, and change the interior framework of the cell and the distribution of action. Also it makes the neural crest cells migrate and division grow during migration,[5,6] so it can promote the migration, differentiation, development and ecesis of the ganglion cells. In this experiment, LN increased significantly in the aganglionic segment, which was different from that in the normal segment (P<0.05). This change may be closely related to the cause of HD.
Aganglionosis occurred in the ls/ls mouse.[7] Abundance of laminin immunoreactivity was found in the terminal colon of the ls/ls mouse, and laminin 1 mRNA increased in the aganglionic colon.[7] Thus the intrinsic defect of the aganglionic region prevented the entry and colonization of the migrating neural crest cells in this portion of the bowel.[8] Unfortunately, the change of laminin mRNA in the aganglionia colon of HD patients was not reported. In our study, LN was far more abundant in the aganglionia colon than in the normal colon of HD patients as shown by immuno-histochemistry staining, and laminin alpha 1 mRNA was also reduced in the aganglionia segment than in the normal segment. RT-PCR is only a qualitative expression, and the result indicates just a tendency. The real-time quantitative PCR allows to change from a qualitative to quantitative determination. It is sensitive to detect the expression of the gene.[9] In this experiment, the expression of laminin alpha 1 in the aganglionic segment was twice that of the normal segment. This finding supports the presumption that the high increase of LN is intrinsic. The abnormal increase of components in the extracellular matrix prevents crest cells from colonizing in the terminal bowel of HD patients and inducing aganglionosis.
We found that the expression of RET gene reduced significantly in the aganglionic colon than in the normal colon at the same time as reported elsewhere.[10,11] The expression tendency of the LN gene and the RET gene differs and there must be some intrinsic relationship between the two phenomena. The mutation of the RET gene and the change of enteric microenvironment represent two different theories.[12] In our experiment, however, the samples were taken from the same patients, and the two genes were detected by the same technique and under the same conditions. Since the expression tendency was divergent, the expression was significantly negative (P<0.001). The mutation of RET, one of the cause of HD,[13] induced the change of transmembrane protein and tyrosine kinase,[14] but it was only found in 40% of patients with HD.[15] Therefore, this theory can not explain the cause of HD in all patients. We suppose that the mutation or expression reduction of RET induces not only the change of tyrosine protein, but also the high expression of the LN gene. The highly increased LN facilitates the early differentiation, development, migration, and ecesis of ganglion cells, which will prevent crest cells from colonizing in the terminal bowel of HD patients. If it were true, the two theories could be unified.
In our study, the expression of LN protein and the LN gene was increased more significantly in the aganglionic colon than in the normal colon in patients with HD. The change of enteric microenvironment contributed to the failure of crest cells to colonize in the terminal bowel of HD patients, while resulting in aganglionosis. At the same time, the expression of the RET gene decreased significantly in the aganglionic colon. Obviously there is an intrinsic relationship between the expression of RET and the development of HD. The high expression of the LN gene and the low expression of the RET gene also indicate an intrinsic relation. Further studies must be done to confirm the relationship between these findings.
Funding: None.
Ethical approval: Not needed.
Competing interest: None.
Contributors: Li AW wrote the first draft of the paper and Zhang WT is the guarantor.
References
1 Martucciello G, Ceccherini I, Lerone M, Jasonni V. Pathogenesis of Hirschsprung's disease. J Pediatr Surg 2000;35:1017-1025.
2 Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst 2005;10:128-143.
3 Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-408.
4 Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat Rec 2001;262:1-15.
5 Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol 2005;21:176-182.
6 Yanagida H, Tanaka J, Maruo S. Immunocytochemical localization of a cell adhesion molecule, integrin alpha5beta1, in nerve growth cones. J Orthop Sci 1999;4:353-360.
7 Rothman TP, Chen J, Howard MJ, Costantini F, Schuchardt A, Pachnis V, et al. Increased expression of laminin-1 and collagen (IV) subunits in the aganglionic bowel of ls/ls, but not c-ret -/- mice. Dev Biol 1996;178:498-513.
8 Payette RF, Tennyson VM, Pomeranz HD, Pham TD, Rothman TP, Gershon MD. Accumulation of components of basal laminae: association with the failure of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice. Dev Biol 1988;125:341-360.
9 Ouyang SY, Yang D, Ouyang HS, Ma HW. The technique and the appliance of real-time polymerase chain reaction. Chemistry Life 2004;24:74-76. (in Chinese)
10 Kusafuka T, Puri P. Altered RET gene mRNA expression in Hirschsprung's disease. J Pediatr Surg 1997;32:600-604.
11 Xu J, Wei MF, Shi HF, Wang G, Li H, Huang SZ. RET mutations in Hirschsprung's disease patients. Chin J Pediatr Surg 1999;20:7-8.
12 Liu H, Chen KN, Zhao YY. The progression of the study of genes correlate to Hirschsprung disease. Int J Genet 2002;25:221-223. (in Chinese)
13 Kjaer S, Ibanez CF. Intrinsic susceptibility to misfolding of a hot-spot for Hirschsprung disease mutations in the ectodomain of RET. Hum Mol Genet 2003;12:2133-2144.
14 Julies MG, Moore SW, Kotze MJ, du Plessis L. Novel RET mutations in Hirschsprung's disease patients from the diverse South African population. Eur J Hum Genet 2001;9:419-423.
15 Gabriel SB, Salomon R, Pelet A, Angrist M, Amiel J, Fornage M, et al. Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat Genet 2002;31:89-93.
Received July 19, 2007 Accepted after revision February 20, 2008
|

