| |
Pediatric reference intervals for soluble
transferrin receptor and transferrin receptor-
ferritin index
Cara Lianne Ooi, Nathalie Lepage, Ed Nieuwenhuys, Ajay Parkash Sharma, Guido Filler
London, Ontario, Canada
Author Affiliations: Department of Pediatrics, Children's Hospital, London Health Science Centre, Schulich School of Medicine & Dentistry, University of Western Ontario, 800 Commissioners Road East, London, Ontario N6A 7W9, Canada (Ooi CL, Sharma AP, Filler G); Department of Pathology and Laboratory Medicine, Children's Hospital of Eastern Ontario, 401 Smyth Road, Ottawa, Canada, K1H 8L1 (Lepage N); Sanquin Diagnostic Services, Amsterdam, The Netherlands (Nieuwenhuys E)
Corresponding Author: Guido Filler, MD, PhD, FRCPC, Department of Pediatrics, Children's Hospital, London Health Science Centre, London, Ontario, Canada, N6A 5W9 (Tel: +1-519-685-8377; Fax: +1-519-685-8551; Email: guido.filler@lhsc.on.ca)doi:10.1007/s12519-009-0024-3
Background: Recent studies showing an improved diagnosis of iron deficiency (ID) with soluble transferrin receptor (sTfR) and sTfR-ferritin index did not take into account the age-dependency of sTfR and ferritin. Moreover, there is a paucity of data on pediatric reference intervals for sTfR and sTfR-ferritin index.
Methods: A study cohort of 436 apparently healthy children was analyzed to establish reference intervals for ferritin, transferrin, sTfR and sTfR-ferritin index. To account for age-dependency, standard deviation scores (Z-scores) for these markers were calculated. The association between these parameters and C-reactive protein (CRP) was analyzed.
Results: The Z-scores of ferritin, transferrin and sTfR had a significant association with CRP, whereas the Z-score of sTfR-ferritin did not correlate with CRP. The reference intervals of these parameters were reported.
Conclusion: Among the different markers of ID, the Z-scores of sTfR, transferrin and ferritin, but not sTfR-ferritin index, associate with the inflammatory status.
Key words: C-reactive protein; ferritin; reference interval; soluble transferrin receptor; soluble transferrin receptor-ferritin index; transferrin
World J Pediatr 2009;5(2):122-126
Introduction
Iron deficiency (ID) is the most common nutritional insufficiency in the world.[1] It has been linked with significant auditory, visual, cognitive, behavioral, motor and immune effects in children.[2] Marrow iron estimation is the current gold standard to assess iron status; however, its invasiveness limits its clinical applicability.[2] Traditionally, serum ferritin, iron and total iron binding capacity are used to diagnose ID. The National Health and Nutrition Examination Study III (NHANES III) defines ID based on the presence of 2 of the following 3 parameters: ferritin <10 ¦Ìg/L, transferrin saturation <10% and erythrocyte protoporphyrin >1.42 ¦Ìmol/L.[3] Iron deficiency anemia is defined as ID plus hemoglobin <110 g/L.[3] The presence of a concomitant inflammation is recognized as affecting the performance of these markers in diagnosing ID.[4,5]
Serum soluble transferrin receptor (sTfR), a monomer lacking 100 amino acids of the transferrin receptor, is another relatively new marker to diagnose ID.[6] A decrease in iron stores upregulates the transferrin receptor, resulting in an increase in serum sTfR levels.[4] Since there is an inverse relationship between sTfR and ferritin in ID, different ratios based on serum sTfR and ferritin levels have been proposed to improve the performance over the either marker.[7-9]
Inflammation can suppress erythropoiesis and hamper the sTfR increase despite the presence of ID.[10] Compared with ferritin and transferrin, sTfR was found to have a better association with ID in chronic infection,[11-13] acute infection,[14] and chronic liver disorders.[15] In spite of a better association, sTfR did not improve the diagnosis of ID in pregnant women with HIV[16] or in general populations.[17,18] Likewise, sTfR did not improve the diagnosis of ID over full blood count and C-reactive protein (CRP) in children with a high burden of infectious diseases.[19] Moreover, serum sTfR has also been reported to have an age-dependent increase in the childhood period.[16] Currently available studies do not account for age-dependency while assessing the relationship between sTfR and inflammation in children. The literature is scarce on the pediatric reference intervals for sTfR and sTfR-ferritin index.
The objectives of this study were to establish pediatric reference intervals for sTfR and sTfR-ferritin index and to assess the relationship of CRP with the Z-scores of sTfR, ferritin, transferrin and the sTfR-ferritin index.
Methods
Two-hundred and sixty patients undergoing minor elective surgeries, including hernia repair (n=33), circumcision (n=8), hypospadia surgery (n=3), orchidopexy (n=10), minor otolaryngologic surgery (n=35), tonsillectomy and/or adenectomy (n=50), minor ophthalmological operation (n=3), minor orthopedic intervention (n=41), minor dental surgery (n=20) and other surgeries (n=57), were identified from the operative record lists at the Children's Hospital of Eastern Ontario (CHEO) and approached by a study nurse at the preoperative anesthesia assessment visit. Written informed consent for the collection of limited anthropometric data and blood sampling was obtained from the parents or from the consenting-minor patients. This cohort was augmented by 176 samples, stored at -20¡ãC, collected for a previous study.[20] Age, gender and the type of surgery were recorded. CHEO's institutional review board gave full approval for the study.
sTfR was measured as previously described.[21] Ferritin, transferrin and CRP were measured by immunonephelometry using BN ProSpec® and the corresponding Dade Behring reagents. The sTfR-ferritin index was calculated as the ratio of sTfR concentration in ¦Ìg/mL over the log of ferritin concentration in ¦Ìg/L as previously described.[22] Wherever possible, simple descriptive statistics was used. Data were tested for normal distribution using the D'Agostino Pearson omnibus normality test. Normally distributed parameters were reported as mean ¡À standard deviation (SD); otherwise, the median and the 2.5th and 97.5th percentiles were recorded. In order to account for age-dependency, we calculated Z-scores for all parameters based on the mean and standard deviation of each age group. The Pearson's correlation coefficient was used for normally distributed data; otherwise, we used the Spearman's rank correlation coefficient to test for a relationship between parameters. All statistical analyses were performed using GraphPad Prism for Windows version 4.01 (GraphPad Software Inc., San Diego, CA, USA), with the exception of the percentiles for the central 95% confidence interval for which SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL, USA) was used.
Results
Four-hundred and thirty-six children (245 males, 191 females) aged 0.4 months to 18 years were studied. As no statistical difference was found between genders for all age groups in all parameters (P>0.05), the data were pooled within the age categories.
Ferritin levels were high in the first 6 months, had a decreasing trend, with a nadir at 18 to 24 months, and a subsequent gradual return to common adult levels in adolescence. Transferrin and sTfR values also showed variation in different age groups.
In most age groups, the data were normally distributed for sTfR and transferrin, whereas the data for ferritin were not normally distributed. In order to report the reference intervals for all parameters, we recorded the median and central 95% reference interval as well as the mean ¡À SD (Table). All these parameters clearly showed an age-dependency, with the non-parametric Spearman's rank correlation coefficient of 0.2278 for ferritin, 0.3066 for transferrin and 0.2390 for sTfR (P<0.0001).
To account for the age-dependency, we calculated the Z-scores for ferritin, transferrin, sTfR and sTfR-ferritin index. The slopes of the regression line between the age and the Z-scores of ferritin, transferrin, sTfR, and sTfR-ferritin index did not significantly differ from zero, indicating that the Z-score calculations introduced no bias (P values between 0.70 and 0.96).
The level of CRP ranged from undetectable values to a maximum of 37.06 mg/L. Although all the patients in this study were asymptomatic and apparently healthy, 17 (3.9%) of them had an elevated CRP level. The distribution of elevated level of CRP was relatively even among the age groups (range, 0.0%-12.8%). The level of CRP was not normally distributed; therefore, the relationship between CRP and the other parameters was analyzed using the non-parametric Spearman's rank correlation coefficient. Ferritin and transferrin both correlated significantly with CRP (Spearman's rank, r=0.1283 and 0.1230; P<0.0085 and P<0.0116). Interestingly, there was a significant correlation between CRP and sTfR (Spearman's rank, r=0.2390, P<0.0001, Fig. 1), and also between CRP and sTfR Z-score (Spearman's rank, r=0.2077, P<0.0001). CRP did not have a significant correlation with sTfR-ferritin index (Spearman's rank, r=0.02790, P=0.5732, Fig. 2) or with sTfR-ferritin index Z-score (Spearman's rank, r=-0.01628, P=0.7424).
Table. Pediatric reference intervals for transferrin, ferritin, soluble transferrin receptor, and the soluble transferrin receptor-ferritin index
|
|
n
|
Median
|
2.5th
percentile |
97.5th
percentile |
Mean ¡À SD
|
|
0.4 to 6 months
|
|
Transferrin (g/L)
|
13
|
2.38
|
1.77
|
6.07
|
2.93¡À1.20
|
|
Ferritin (¦Ìg/L)
|
13
|
102.66
|
3.28
|
336.34
|
148.45¡À106.84
|
|
sTfR (mg/L)
|
13
|
1.52
|
1.26
|
3.17
|
1.78¡À0.58
|
|
sTfR/log(ferritin)
|
13
|
0.74
|
0.57
|
4.81
|
1.25¡À1.29
|
|
6 to 12 months
|
|
Transferrin (g/L)
|
17
|
2.82
|
1.04
|
3.83
|
2.74¡À0.65
|
|
Ferritin (¦Ìg/L)
|
17
|
31.61
|
5.04
|
56.55
|
29.31¡À14.51
|
|
sTfR (mg/L)
|
17
|
1.66
|
1.12
|
2.91
|
1.75¡À0.41
|
|
sTfR/log(ferritin)
|
17
|
1.22
|
0.81
|
2.96
|
1.36¡À0.57
|
|
12 to 18 months
|
|
Transferrin (g/L)
|
16
|
3.27
|
2.02
|
4.31
|
3.19¡À0.66
|
|
Ferritin (¦Ìg/L)
|
16
|
13.29
|
6.48
|
102.03
|
30.38¡À30.05
|
|
sTfR (mg/L)
|
16
|
1.89
|
1.37
|
2.52
|
1.90¡À0.39
|
|
sTfR/log(ferritin)
|
16
|
1.51
|
0.82
|
2.70
|
1.58¡À0.54
|
|
18 months to 2 years
|
|
Transferrin (g/L)
|
21
|
3.15
|
2.22
|
4.06
|
3.06¡À0.45
|
|
Ferritin (¦Ìg/L)
|
21
|
17.82
|
8.89
|
53.88
|
22.22¡À12.69
|
|
sTfR (mg/L)
|
20
|
1.72
|
1.33
|
2.93
|
1.82¡À0.39
|
|
sTfR/log(ferritin)
|
20
|
1.46
|
0.97
|
2.34
|
1.45¡À0.34
|
|
2 to 3 years
|
|
Transferrin (g/L)
|
47
|
2.73
|
1.96
|
4.26
|
2.83¡À0.52
|
|
Ferritin (¦Ìg/L)
|
47
|
17.36
|
2.55
|
81.29
|
21.80¡À15.74
|
|
sTfR (mg/L)
|
46
|
1.70
|
0.98
|
2.91
|
1.71¡À0.41
|
|
sTfR/log(ferritin)
|
46
|
1.32
|
0.65
|
5.55
|
1.61¡À1.01
|
|
3 to 4 years
|
|
Transferrin (g/L)
|
39
|
2.76
|
1.76
|
4.13
|
2.81¡À0.56
|
|
Ferritin (¦Ìg/L)
|
39
|
26.26
|
3.14
|
192.00
|
36.94¡À38.54
|
|
sTfR (mg/L)
|
38
|
1.57
|
1.08
|
2.55
|
1.63¡À0.34
|
|
sTfR/log(ferritin)
|
38
|
1.19
|
0.67
|
4.29
|
1.28¡À0.64
|
|
4 to 6 years
|
|
Transferrin (g/L)
|
76
|
2.69
|
2.04
|
4.78
|
2.88¡À0.72
|
|
Ferritin (¦Ìg/L)
|
76
|
23.98
|
5.26
|
108.98
|
31.34¡À23.48
|
|
sTfR (mg/L)
|
76
|
1.50
|
1.10
|
2.74
|
1.61¡À0.42
|
|
sTfR/log(ferritin)
|
76
|
1.12
|
0.69
|
2.34
|
1.22¡À0.41
|
|
6 to 9 years
|
|
Transferrin (g/L)
|
64
|
2.55
|
2.05
|
4.86
|
2.70¡À0.56
|
|
Ferritin (¦Ìg/L)
|
58
|
27.83
|
3.61
|
78.90
|
29.99¡À16.33
|
|
sTfR (mg/L)
|
64
|
1.40
|
0.93
|
2.63
|
1.50¡À0.37
|
|
sTfR/log(ferritin)
|
58
|
0.99
|
0.60
|
5.26
|
1.18¡À0.88
|
|
9 to 12 years
|
|
Transferrin (g/L)
|
44
|
2.64
|
1.86
|
4.19
|
2.70¡À0.51
|
|
Ferritin (¦Ìg/L)
|
44
|
31.29
|
4.84
|
242.21
|
40.20¡À38.71
|
|
sTfR (mg/L)
|
44
|
1.42
|
0.81
|
2.67
|
1.46¡À0.32
|
|
sTfR/log(ferritin)
|
44
|
0.94
|
0.35
|
2.27
|
1.03¡À0.35
|
|
12 to 18 years
|
|
Transferrin (g/L)
|
86
|
2.79
|
2.08
|
4.00
|
2.85¡À0.49
|
|
Ferritin (¦Ìg/L)
|
86
|
29.39
|
2.76
|
108.86
|
36.79¡À23.78
|
|
sTfR (mg/L)
|
85
|
1.34
|
0.91
|
1.91
|
1.37¡À0.24
|
|
sTfR/log(ferritin)
|
85
|
0.92
|
0.67
|
3.05
|
1.00¡À0.46
|
sTfR: soluble transferrin receptor.
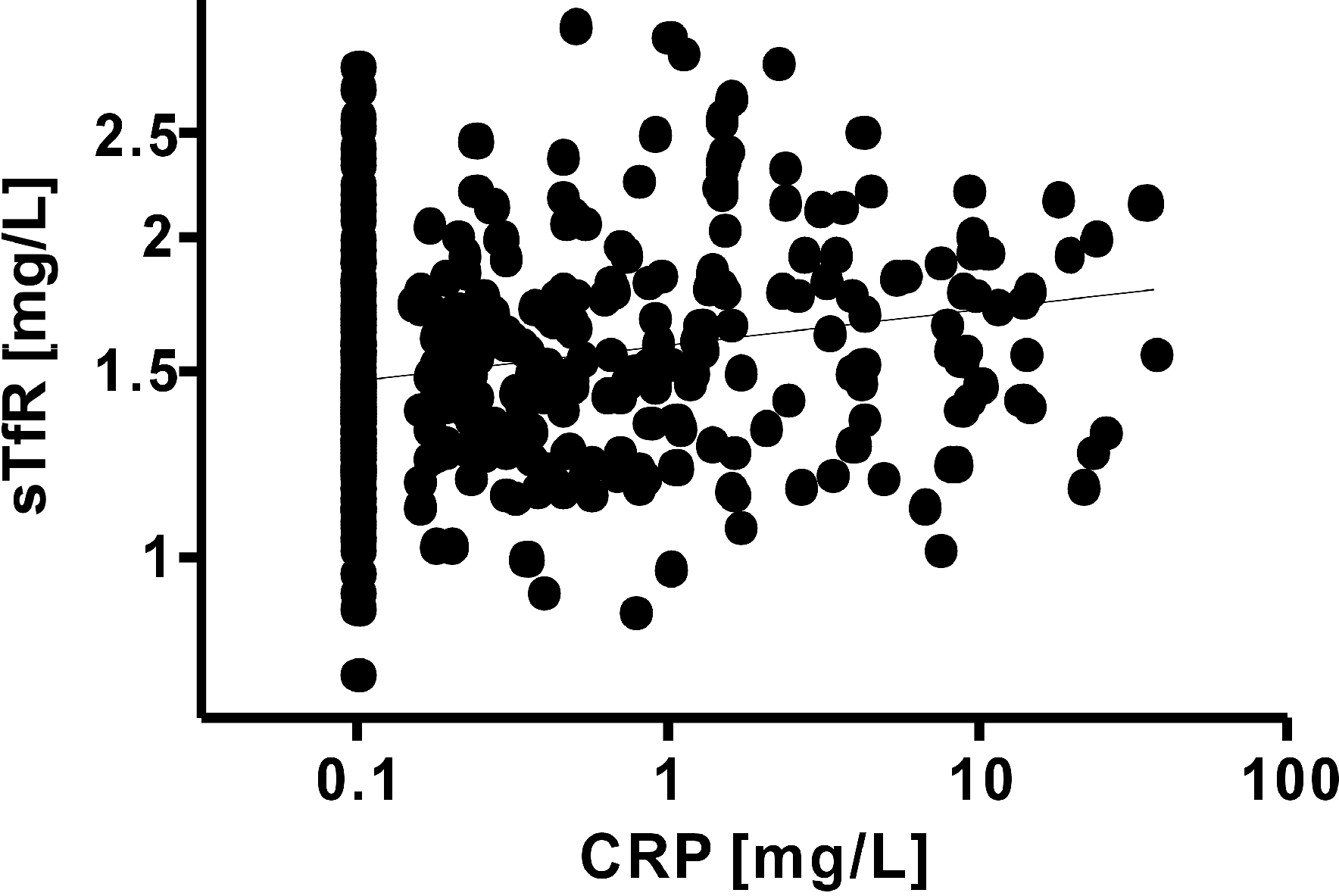
Fig. 1. The relationship between soluble transferrin receptor (sTfR) concentration and C-reactive protein (CRP) in 436 apparently healthy children. There was a significant correlation between the two parameters (Spearman's rank, r=0.2390, P<0.0001).
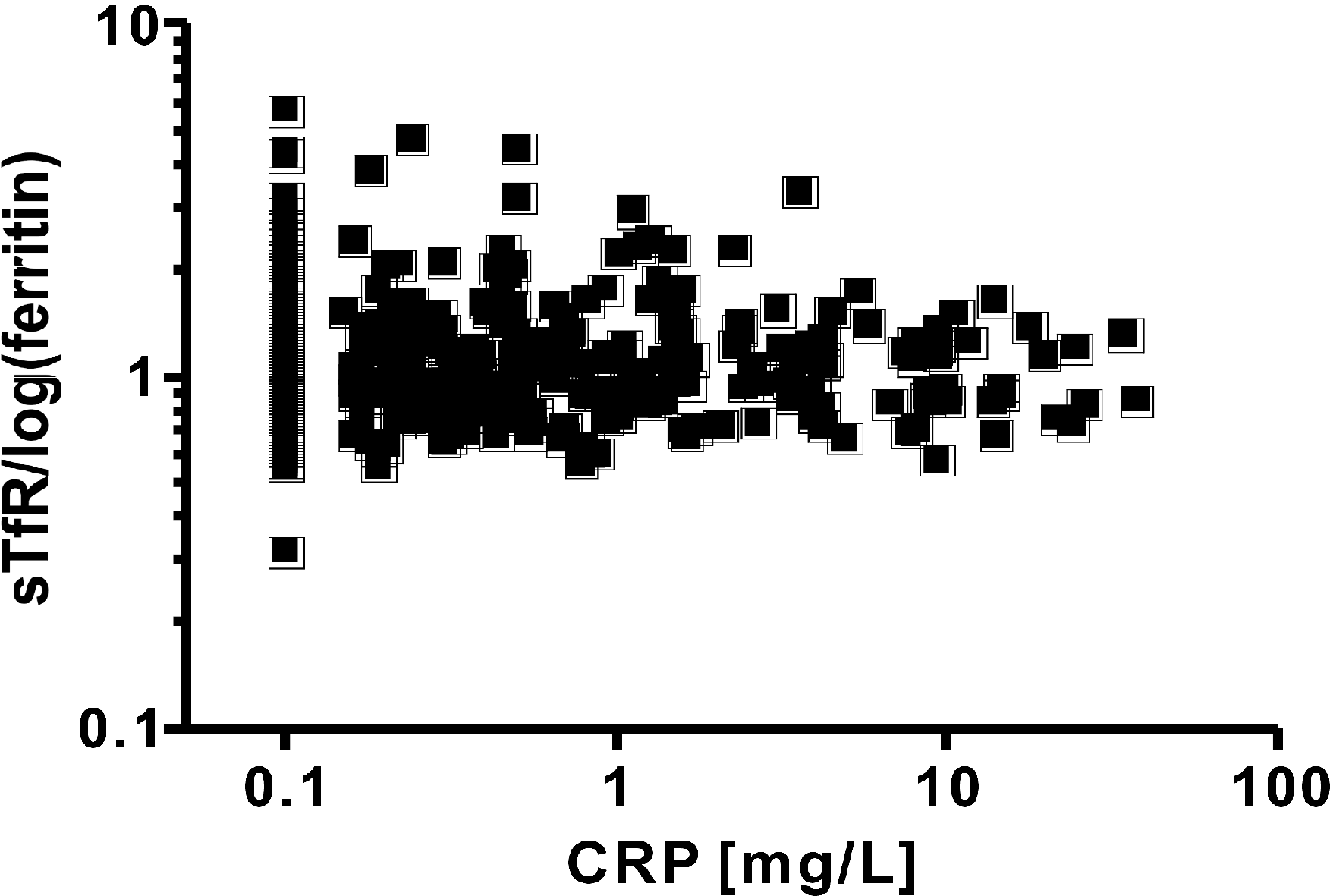 Fig. 2. The relationship between the soluble transferrin ferritin index [sTfR/log(ferritin)] and C-reactive protein (CRP) in 436 apparently healthy children. There was no significant correlation between the two parameters. Fig. 2. The relationship between the soluble transferrin ferritin index [sTfR/log(ferritin)] and C-reactive protein (CRP) in 436 apparently healthy children. There was no significant correlation between the two parameters.
Discussion
The main objectives of this study were to establish sTfR and sTfR-ferritin index reference intervals for the new Dade Behring assay and to assess the relationship of CRP with sTfR, ferritin, transferrin and sTfR-ferritin index.
There is a paucity of data on pediatric reference intervals for sTfR and sTfR-ferritin index. We are unaware of any previous publications on pediatric reference intervals for sTfR using the Dade Behring assay. Kratovil et al[23] published age-dependent reference intervals in 183 children, using the Quantikine IVD sTfR Immunoassay kit. Their reference ranges reported were 1.37-2.85 mg/L for 6 to 24 months, 1.05-3.05 mg/L for 2 to 6 years, 1.16-2.72 mg/L for 7 to 12 years, 0.97-2.60 mg/L for 13 to 17 years, and 0.84-2.32 mg/L for ¡Ý18 years.[23] Our data compared favorably with these reference intervals. Additionally, we reported the reference ranges under the age of 6 months, and expanded on other age groups from a larger study cohort. Malope et al[22] reported in a study with sTfR measured by enzyme-linked immunosorbent assay a Log sTfR: ferritin ratio >2.55 for ID and <2.55 for anemia of inflammation as the cut-off to diagnose ID in children aged 1-6 years. Our results have a reasonable agreement with their cut-offs; however, we observed an age-dependency of sTfR-ferritin index, similar to that of sTfR observed by Kratovil et al.[23]
The utility of sTfR in diagnosing ID in the presence of inflammation has been debated lately. To evaluate this issue further, we assessed the relationship of CRP with sTfR and sTfR-ferritin index in apparently healthy children. We also measured the traditional markers of ID, transferrin and ferritin in our study cohort. To improve on previous studies, we accounted for the age dependency of these parameters by calculating their Z-scores.
In our study sample, CRP had a significant association with ferritin as well as with transferrin. Ferritin is a recognized positive acute phase reactant, whereas inflammation decreases transferrin production. The ferritin peak observed in the first 6 months was consistent with previously reported high ferritin at birth and early infancy.[24]
While assessing the effect of inflammation on sTfR, sTfR showed a positive association with CRP. This association persisted even after accounting for the age-dependency of sTfR. As is already known, ID stimulates an increase in sTfR levels due to the compensatory increase in erythropoiesis.[6-12] Conversely, inflammation can also decrease sTfR production, thus lowering its serum level in rheumatoid arthritis,[25] inflammatory bowel disease,[26] and other inflammatory disorders.[27] A similar acute phase reactant potential of sTfR was also reported in children with a high load of infection and inflammation.[18] Because there is an opposing effect of ID and inflammation on sTfR production, the relative severity of ID and inflammation can affect the relationship between sTfR and CRP in a study sample.
To explore this relationship further, we evaluated the association between CRP and sTfR-ferritin index. Based on an increase in sTfR level from stimulated erythropoiesis and a decrease in ferritin levels with reduced iron stores, an sTfR-ferritin ratio has been proposed to be a better marker for ID than either sTfR or ferritin alone.[27] In the presence of inflammation, the acute-phase decrease in sTfR level and an opposite increase in serum ferritin can affect the diagnostic accuracy of this ratio. In our study cohort, sTfR-ferritin index did not have a significant association with CRP, even though CRP correlated significantly with sTfR and ferritin individually. Possibly this could be due to the reverse direction of the change in sTfR and ferritin with inflammation. Previous studies have also shown a better performance of sTfR-ferritin index over either sTfR or ferritin in diagnosing ID in the presence of inflammation.[22,28] The statistically insignificant association between sTfR-ferritin index and CRP, even after calculating the respective Z-scores, was the novel finding from our study. This observation suggests an independence of sTfR-ferritin index from inflammation even after accounting for the age-dependency of these variables.
Our study has a few limitations. Instead of a study sample from the community at large, we enrolled our subjects during a hospital visit. The inclusion prior to an elective minor procedure minimized significant confounding from a major comorbidity on our reference intervals. Otherwise, all included subjects were clinically asymptomatic and the incidence of elevated CRP was low (3.9%). Understandably, marrow iron testing improves the quantification of the iron status. Considering the primary focus of our study, the lack of marrow iron quantification should not alter the relative relationship among the studied ID indices and CRP. The screening investigations also excluded any suggestion of hemolysis to affect the sTfR levels. Considering the selection of apparently healthy children, our results cannot be extrapolated to the inflammatory states.
In summary, inflammation associates significantly with sTfR, ferritin and transferrin in healthy children, even after accounting for the age-dependency of these variables. sTfR-ferritin index appears to be independent from inflammation. The applicability of our findings to the patients with inflammatory conditions needs further validation.
Funding: The study was supported by grants from Dade Behring GmbH, Marburg, Germany and Dade Behring Inc., Mississauga, ON, Canada.
Ethical approval: The Institutional Review Board of the Children's Hospital of Eastern Ontariogave gave full approval for the study.
Competing interest: None.
Contributors: Lepage N, Niewenhuys E and Filler G designed and executed the study. Ooi CL and Filler G performed the analysis and wrote the manuscript and it was critically edited and revised by the other two co-authors. Sharma AP and Filler G prepared the final version.
References
1 Soldin OP, Bierbower LH, Choi JJ, Choi JJ, Thompson-Hoffman S, Soldin SJ. Serum iron, ferritin, transferrin, total iron binding capacity, hs-CRP, LDL cholesterol and magnesium in children; new reference intervals using the Dade Dimension Clinical Chemistry System. Clin Chim Acta 2004;342:211-217.
2 Moy RJ. Prevalence, consequences and prevention of childhood nutritional iron deficiency: a child public health perspective. Clin Lab Haematol 2006;28:291-298.
3 Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973-976.
4 Cook JD, Skikne BS, Baynes RD. Serum transferrin receptor. Annu Rev Med 1993;44:63-74.
5 Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 2003;329:9-22.
6 Virtanen MA, Viinikka LU, Virtanen MK, Svahn JC, Anttila RM, Krusius T, et al. Higher concentrations of serum transferrin receptor in children than in adults. Am J Clin Nutr 1999;69:256-260.
7 Cermak J, Brabec V. Transferrin receptor-ferritin index: a useful parameter in differential diagnosis of iron deficiency and hyperplastic erythropoiesis. Eur J Haematol 1998;61:210-212.
8 Vazquez Lopez MA, Carracedo A, Lendinez F, Munoz FJ, Lopez J, Munoz A. The usefulness of serum transferrin receptor for discriminating iron deficiency without anemia in children. Haematologica 2006;91:264-265.
9 Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92:2934-2939.
10 Kasvosve I, Gomo ZA, Nathoo KJ, Matibe P, Mudenge B, Gordeuk VR. Reference intervals of serum transferrin receptors in pre-school children in Zimbabwe. Clin Chim Acta 2007;382:138-141.
11 Asobayire FS, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Cote d'Ivoire. Am J Clin Nutr 2001;74:776-782.
12 Lammi-Keefe CJ, Lickteig ES, Ahluwalia N, Haley NR. Day-to-day variation in iron status indexes is similar for most measures in elderly women with and without rheumatoid arthritis. J Am Diet Assoc 1996;96:247-351.
13 Ferguson BJ, Skikne BS, Simpson KM, Baynes RD, Cook JD. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med 1992;119:385-390.
14 Dimitriou H, Stiakaki E, Markaki EA, Bolonaki I, Giannakopoulou C, Kalmanti M. Soluble transferrin receptor levels and soluble transferrin receptor/log ferritin index in the evaluation of erythropoietic status in childhood infections and malignancy. Acta Paediatr 2000;89:1169-1173.
15 Reynolds P. Newborns have unique confounding factors regarding the TfR-F ratio. Arch Dis Child Fetal Neonatal Ed 2001;85:F146.
16 Semba RD, Kumwenda N, Hoover DR, Taha TE, Mtimavalye L, Broadhead R, et al. Assessment of iron status using plasma transferrin receptor in pregnant women with and without human immunodeficiency virus infection in Malawi. Eur J Clin Nutr 2000;54:872-877.
17 Wians FH Jr, Urban JE, Keffer JH, Kroft SH. Discriminating between iron deficiency anemia and anemia of chronic disease using traditional indices of iron status vs. transferrin receptor concentration. Am J Clin Pathol 2001;115:112-118.
18 Wians FH Jr, Urban JE, Kroft SH, Keffer JH. Soluble transferrin receptor (sTfR) concentration quantified using two sTfR kits: analytical and clinical performance characteristics. Clin Chim Acta 2001;303:75-81.
19 Ritchie B, McNeil Y, Brewster DR. Soluble transferrin receptor in Aboriginal children with a high prevalence of iron deficiency and infection. Trop Med Int Health 2004;9:96-105.
20 Vlug A, Nieuwenhuys EJ, van Eijk RV, Geertzen HG, van Houte AJ. Nephelometric measurements of human IgG subclasses and their reference ranges. Ann Biol Clin (Paris) 1994;52:561-567.
21 Abellan R, Ventura R, Pichini S, Sarda MP, Remacha AF, Pascual JA, et al. Evaluation of immunoassays for the measurement of soluble transferrin receptor as an indirect biomarker of recombinant human erythropoietin misuse in sport. J Immunol Methods 2004;295:89-99.
22 Malope BI, MacPhail AP, Alberts M, Hiss DC. The ratio of serum transferrin receptor and serum ferritin in the diagnosis of iron status. Br J Haematol 2001;115:84-89.
23 Kratovil T, DeBerardinis J, Gallagher N, Luban NL, Soldin SJ, Wong EC. Age specific reference intervals for soluble transferrin receptor (sTfR). Clin Chim Acta 2007;380:222-224.
24 Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology 2007;92:73-82.
25 Zoli A, Altomonte L, Mirone L, Magar¨® M, Ricerca BM, Storti S, et al. Serum transferrin receptors in rheumatoid arthritis. Ann Rheum Dis 1994;53:699-701.
26 Revel-Vilk S, Tamary H, Broide E, Zoldan M, Dinari G, Zahavi I, et al. Serum transferrin receptor in children and adolescents with inflammatory bowel disease. Eur J Pediatr 2000;159:585-589.
27 Junc¨¤ J, Fern¨¢ndez-Avil¨¦s F, Oriol A, Navarro JT, Mill¨¢ F, Sancho JM, et al. The usefulness of the serum transferrin receptor in detecting iron deficiency in the anemia of chronic disorders. Haematologica 1998;83:676-680.
28 Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997;89:1052-1057.
Received July 7, 2008 Accepted after revision February 19, 2009
|

